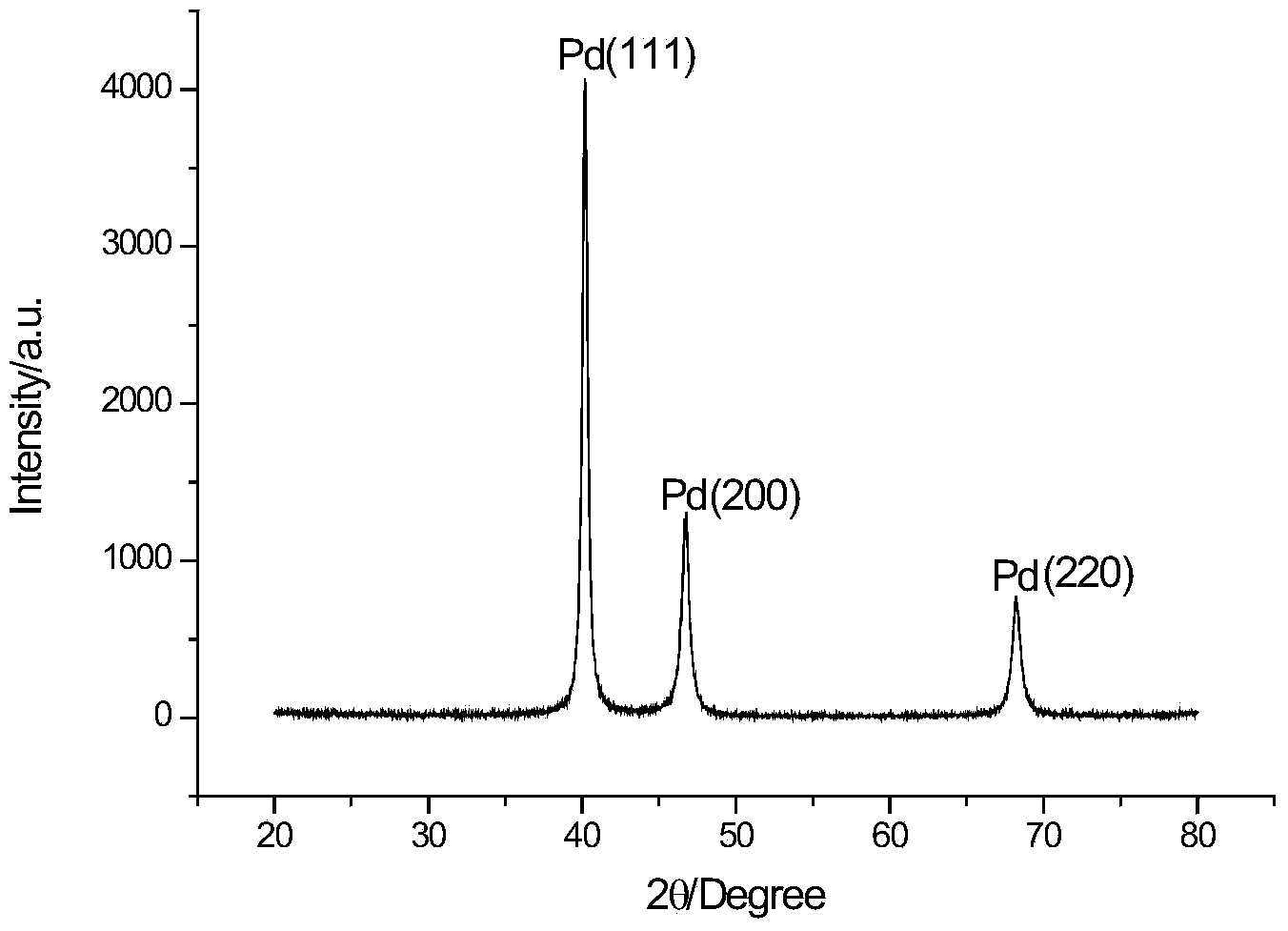
Method for electrodepositing palladium from ionic liquid
An ionic liquid and electrodeposition technology, applied in the field of environmentally friendly electrochemical technology and high efficiency, can solve the problems of long electrodeposition time, increased energy consumption and high cost, and achieve the effects of simple operation process, avoiding energy consumption and low equipment cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Weigh 0.05mol of 1-ethyl-3-methylimidazolium trifluoroacetate (EMIMCF3 COO) ionic liquid.
[0035] (2) to EMIMCF 3 Add 0.2gPdCl to COO 2 , under constant temperature magnetic stirring for 30min at 25°C, the PdCl 2 To reach a supersaturated state, the purpose is to avoid the continuous consumption of Pd during the constant current electrodeposition process. 2+ , such that Pd 2+ It cannot diffuse around the cathode in time, resulting in an increase in the degree of concentration polarization while maintaining a constant current.
[0036] (3) Perform cyclic voltammetry curve test on the mixed system to obtain the reduction peak of palladium ions, which corresponds to the current of palladium ions reduced to elemental palladium, and the calculated current density is 0.02mA / mm 2 .
[0037] (4) Calculate the current of the electrodeposition process according to the current density in (3) and the surface area of the cathode used in the electrodeposition, and conduct...
Embodiment 2
[0041] (1) Weigh 0.05mol 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (EMIMCF 3 SO 3 ) ionic liquids.
[0042] (2) to EMIMCF 3 SO 3 Add 0.2gPdCl to 2 , under constant temperature magnetic stirring for 30min at 35°C, the PdCl 2 To reach a supersaturated state, the purpose is to avoid the continuous consumption of Pd during the constant current electrodeposition process. 2+ , such that Pd 2+ It cannot diffuse around the cathode in time, resulting in an increase in the degree of concentration polarization while maintaining a constant current.
[0043] (3) Perform cyclic voltammetry curve test on the mixed system to obtain the reduction peak of palladium ions, which corresponds to the current of palladium ions reduced to elemental palladium, and the calculated current density is 0.016mA / mm 2 .
[0044] (4) Calculate the current of the electrodeposition process according to the current density in (3) and the surface area of the cathode used in the electrodepositi...
Embodiment 3
[0048] (1) Weigh 0.05mol 1-ethyl-3-methylimidazolium hexafluorophosphate (EMIMPF 6 ) ionic liquids.
[0049] (2) Heating up to 70°C, at this time EMIMPF 6 has completely melted into a liquid state, then add 0.2g of PdCl to it 2 , under constant temperature magnetic stirring at 70°C for 1h, the PdCl 2 To reach a supersaturated state, the purpose is to avoid the continuous consumption of Pd during the constant current electrodeposition process. 2+ , such that Pd 2+ It cannot diffuse around the cathode in time, resulting in an increase in the degree of concentration polarization while maintaining a constant current.
[0050] (3) Perform cyclic voltammetry curve test on the mixed system to obtain the reduction peak of palladium ions, which corresponds to the current of palladium ions reduced to elemental palladium, and the calculated current density is 0.03mA / mm 2 .
[0051] (4) Calculate the current of the electrodeposition process according to the current density in (3) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com