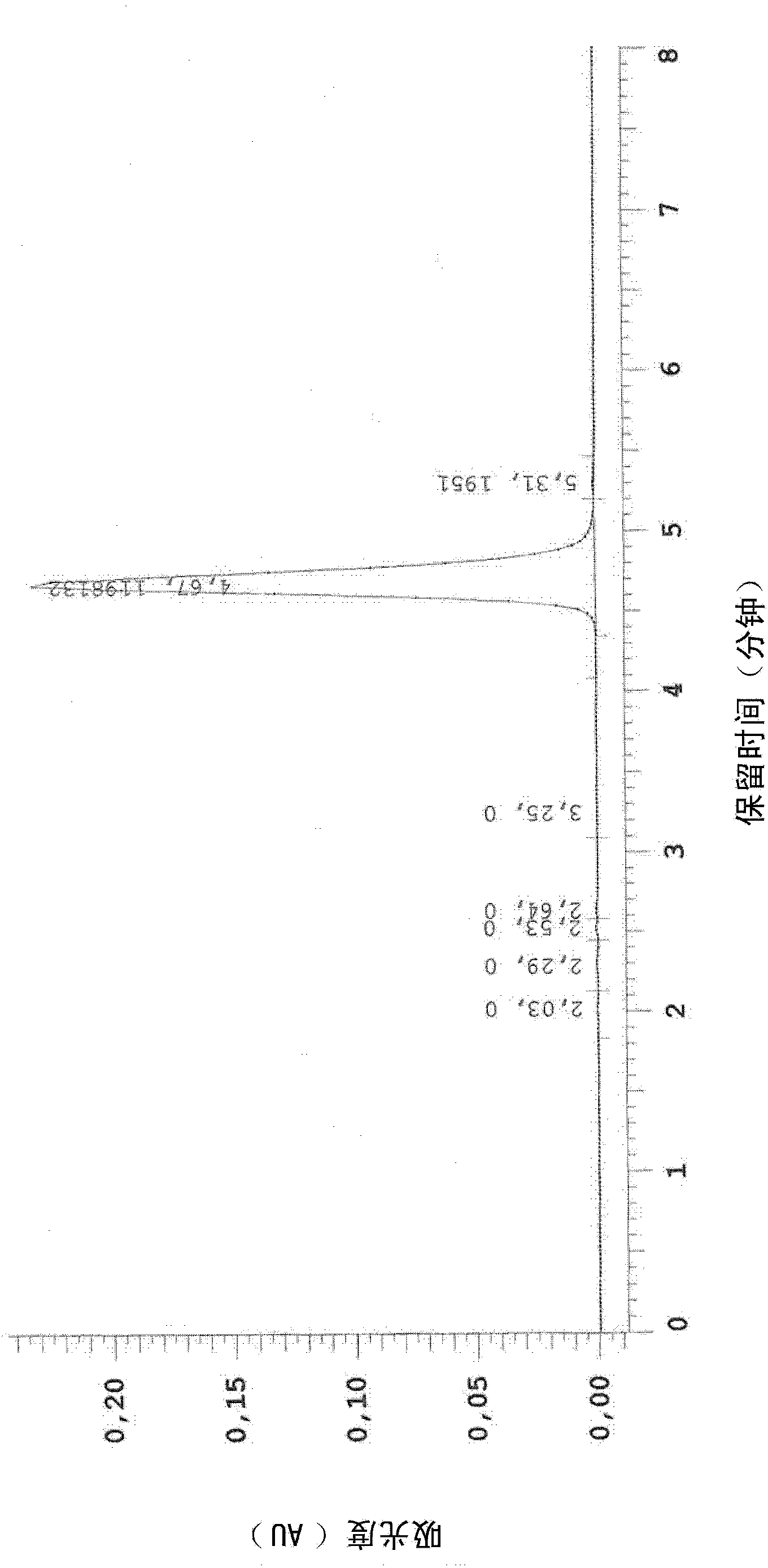
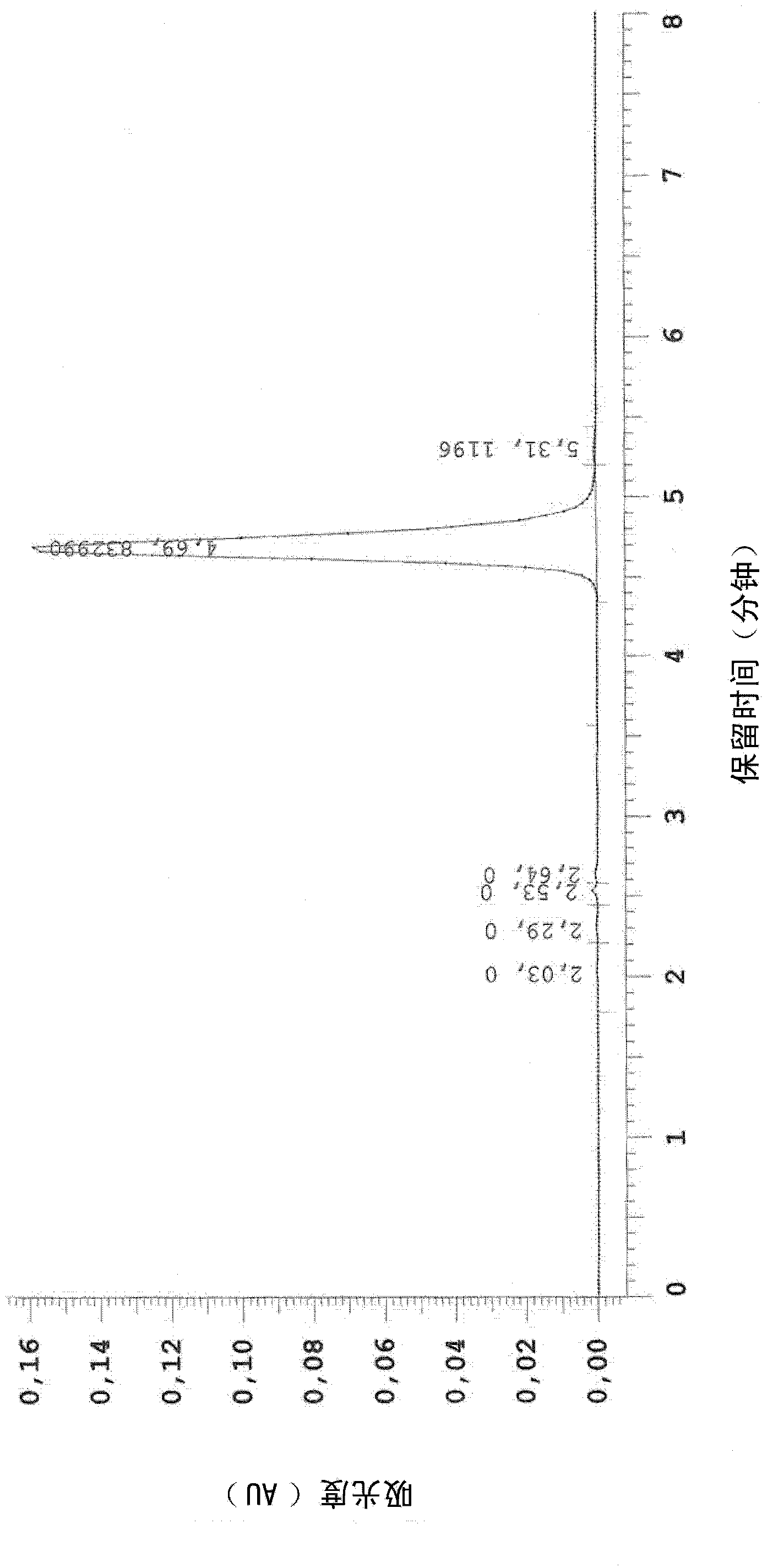
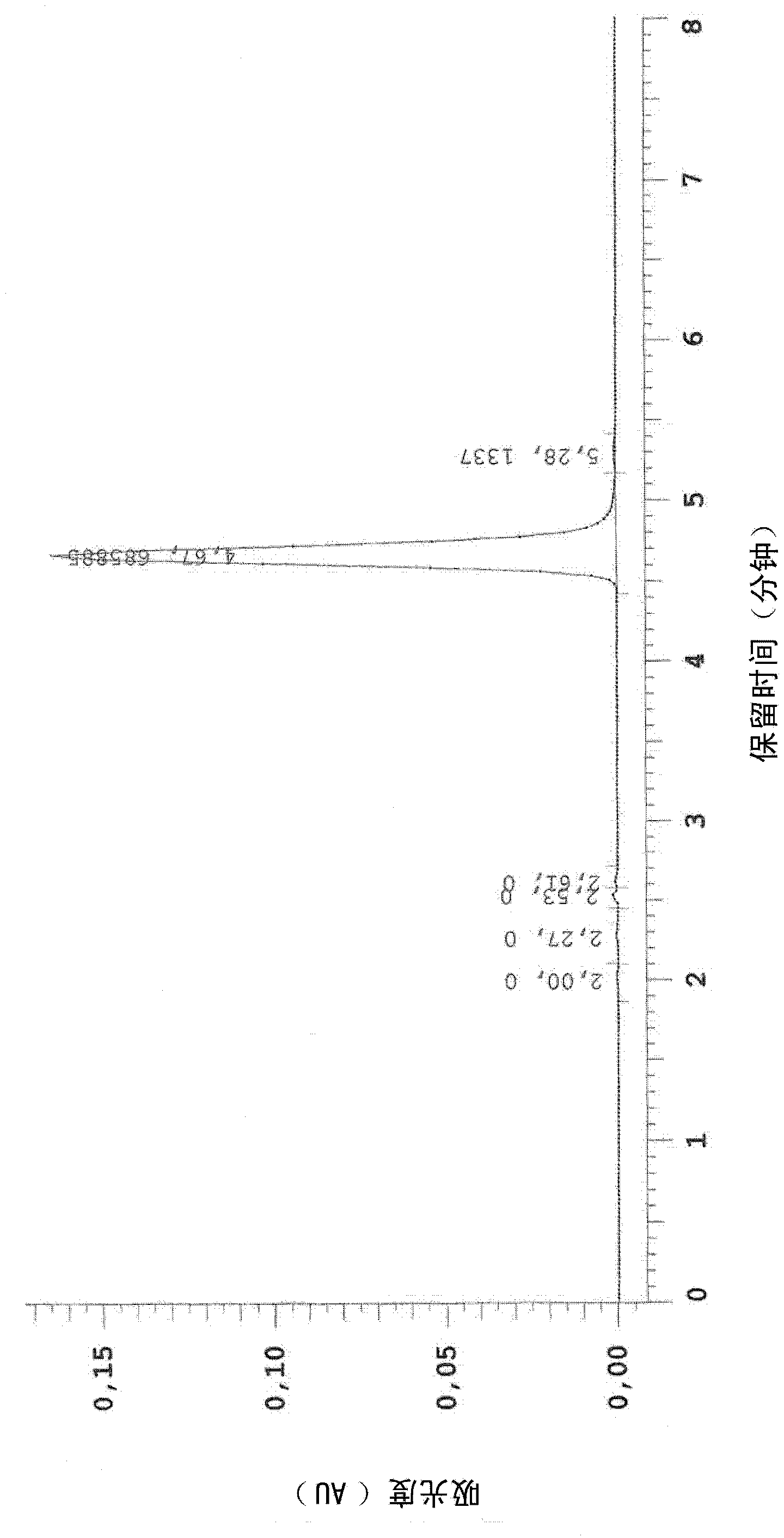Composition and method for treating hpv
A composition and drug technology, applied in the field of treatment of viral infections, can solve the problems of activity decline and low stability of cidofovir, and achieve the effects of good stability and elimination of the risk of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Embodiment 1: freeze-drying condition
[0124] Lyophilization of the aqueous composition comprising a bioadhesive polymer, and optionally a plasticizer and / or cidofovir to obtain a porous extensible matrix is carried out according to the following protocol.
[0125] The polymer was dispersed in distilled water with slow stirring until completely homogeneous. Optionally, the dispersion obtained is stirred again after addition of the plasticizer until completely homogeneous. Disperse cidofovir and add 2M NaOH stock solution to reach pH7. The obtained mixture was transferred to a crystallizer and lyophilized. The freeze-drying conditions are as follows:
[0126] freezing
[0127] stage
temperature(°C)
time (h)
pressure (bar)
1
-35 (-30 to -35)
3.0 (2 to 4)
surroundings
2
-35 (-30 to -35)
0.5 (0.4 to 0.6)
surroundings
[0128] primary drying
[0129] stage
temperature(°C)
time (h) ...
Embodiment 2
[0132] Example 2: Components of the lyophilized composition
[0133] The following components and mixtures were evaluated for their ability to form the desired "sponge" structure after lyophilization.
[0134] Bioadhesive polymers:
[0135] -Hydroxypropylmethylcellulose (HPMC)
[0136] HPMC E5: Viscosity: 5mPa.s (=5cp) (2% aqueous solution)
[0137] HPMC E15: Viscosity: 12-18mPa.s (2% aqueous solution)
[0138] HPMC4000: Viscosity: 4000-5600mPa.s (2% aqueous solution)
[0139] HPMC K15: Viscosity: 11250-21000mPa.s (2% aqueous solution)
[0140] - Sodium Carboxymethyl Cellulose (NaCMC)
[0141] -Hydroxyethylcellulose (HEC)
[0142] HEC Natrosol250HX: Viscosity: 1500-2500mPa.s (1% aqueous solution)
[0143] HEC Natrosol250HHX: Viscosity: 3500-5500mPa.s (1% aqueous solution)
[0144] HEC Natrosol250M: Viscosity: 4500-6500mPa.s (2% aqueous solution)
[0145] HEC H4000: Viscosity: 4500-6500mPa.s (2% aqueous solution)
[0146] -Carbomer (Carbomer) 974P
[0147] -Hydroxypr...
Embodiment 3
[0155] Example 3: Evaluation of different lyophilized placebo compositions
[0156] Components were tested at different conditions and concentrations to assess the desired properties of the lyophilizate. A desired characteristic is a spongy texture that is easily spreadable when dry and capable of rehydrating easily and / or quickly to a gel of moderate viscosity (ie, not too liquid-like and not too viscous).
[0157] The following conditions were kept constant for easy comparison: crystallizer diameter (4 cm), the amount of water used to disperse the components (add 6 g (ad6 g), meaning that water was added to the composition until 6 g of the final composition before lyophilization), and Freeze drying cycle.
[0158] The placebo lyophilisate (ie without cidofovir) was evaluated first. Table 1 lists the tested concentrations of polymers and plasticizers.
[0159] Table 1
[0160] polymer
mg / cm 2
plasticizer
mg / cm 2
Remark
[0161] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com