Lovastatin capsule and preparation method thereof
A technology of lovastatin and capsules, applied in the field of lovastatin capsules and its preparation, which can solve the problems of hard tablets and inability to disintegrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
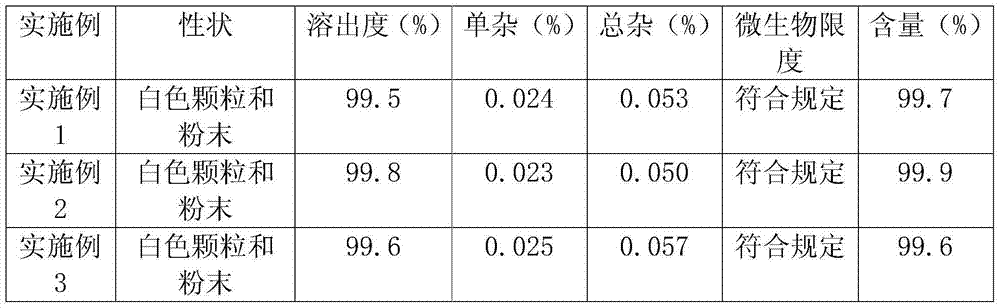
Embodiment 1
[0066] prescription:
[0067] Lovastatin 20g
[0068] Low-substituted hydroxypropyl cellulose 50g
[0069] Crospovidone 5g
[0070] Sodium Lauryl Sulfate 2g
[0071] Povidone K30 5g
[0073] Makes 1000 capsules
[0074] Preparation:
[0075] (1) Adhesive preparation: take by weighing sodium lauryl sulfate and povidone K30 of recipe quantity, add water to dissolve, be mixed with the solution of 10% povidone K30;
[0076] (2) Sieving: Excipients low-substituted hydroxypropyl cellulose, crospovidone and magnesium stearate were respectively passed through a 60-mesh sieve; (3) Granulation and drying: Weighed the prescription amount of lovastatin raw material and mixed In the machine, add the above-mentioned adhesive to make soft materials, and then use the granulator to granulate; after the wet granules are dried at 40°C to 45°C for 1 to 3 hours, the granules are sized;
[0077] (4) Mixing: put the above-mentioned granules in a mixer, add low-...
Embodiment 2
[0081] prescription:
[0082] Lovastatin 20g
[0083] Low-substituted hydroxypropyl cellulose 200g
[0084] Crospovidone 5g
[0085] Sodium Lauryl Sulfate 5g
[0086] Povidone K30 10g
[0088] Makes 1000 capsules
[0089] The preparation method is the same as in Example 1.
Embodiment 3
[0091] prescription:
[0092] Lovastatin 20g
[0093] Low-substituted hydroxypropyl cellulose 150g
[0094] Crospovidone 2.5g
[0095] Sodium Lauryl Sulfate 3g
[0096] Povidone K30 7.5g
[0098] Makes 1000 capsules
[0099] The preparation method is the same as in Example 1.
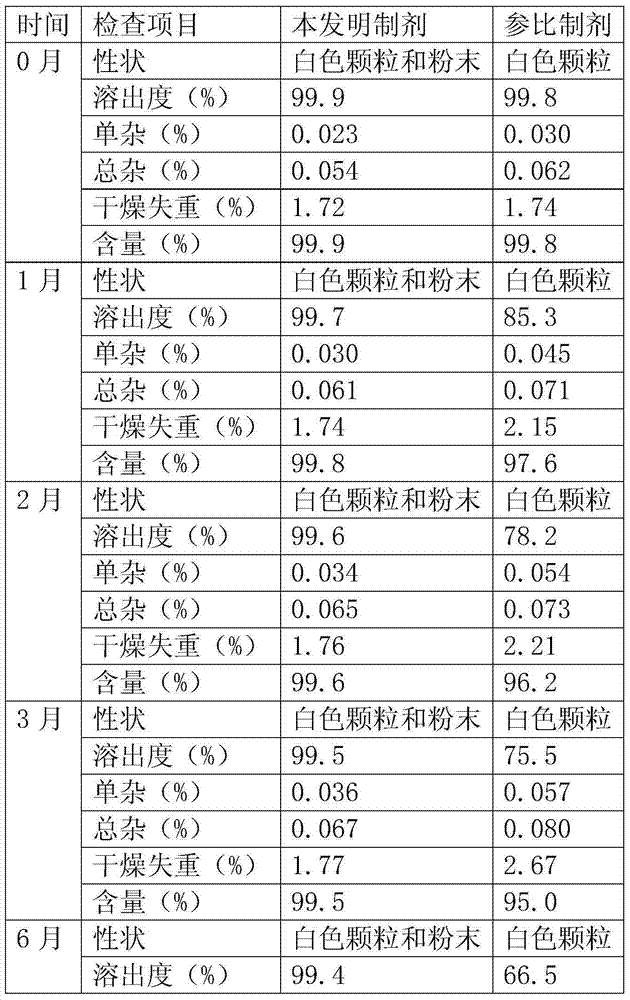
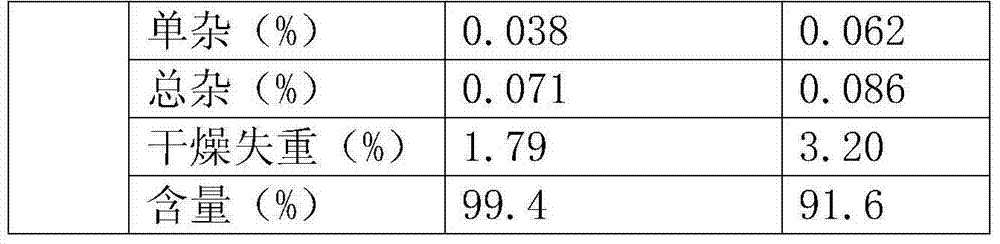
[0100] biological test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com