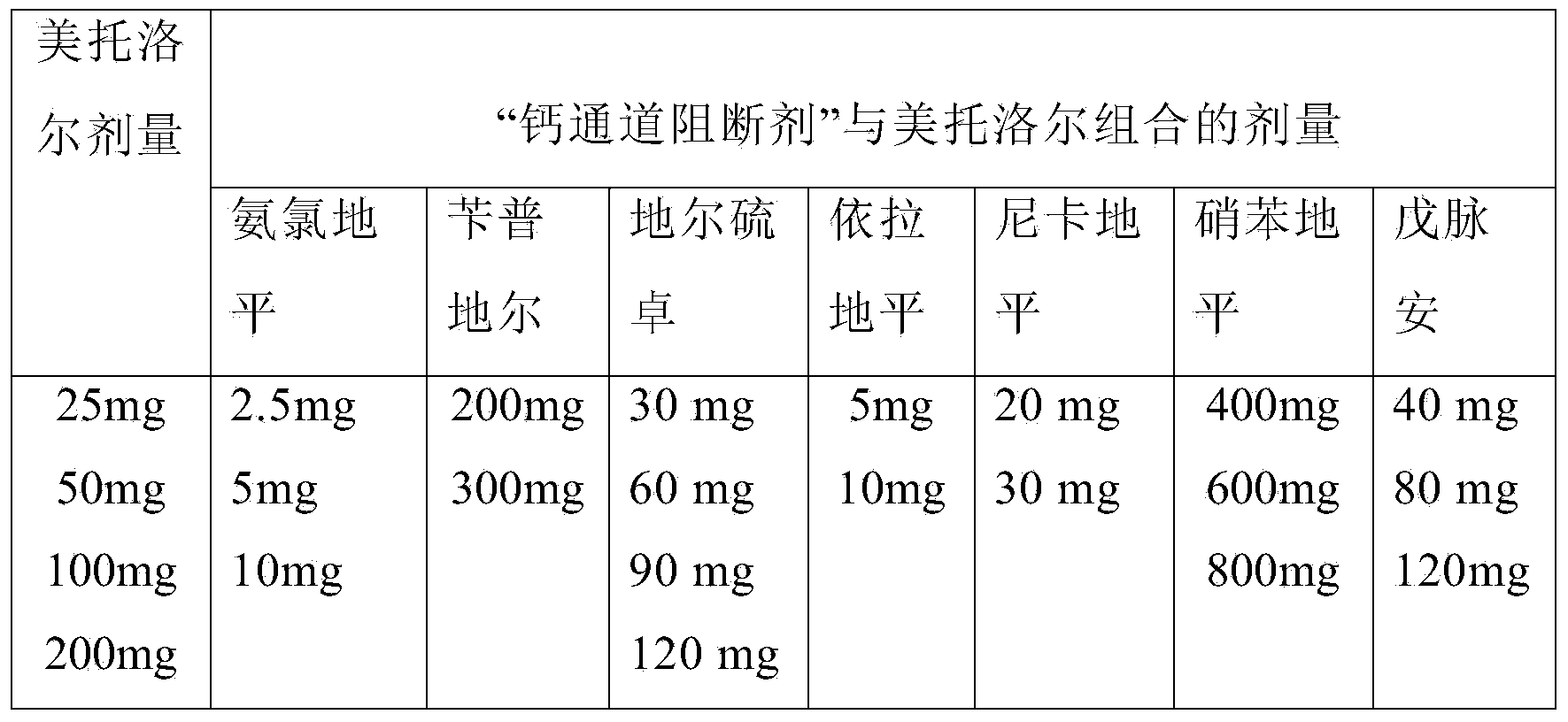
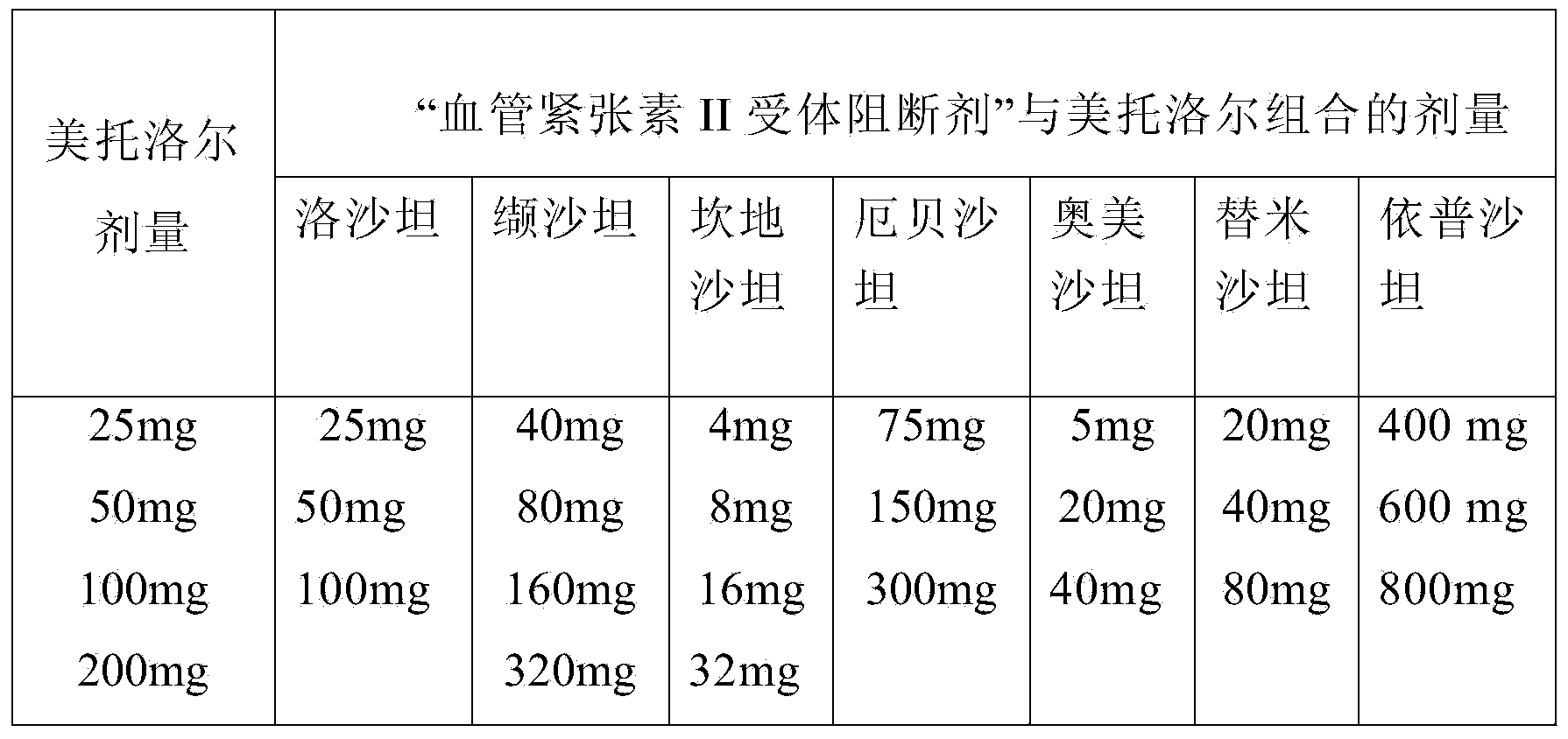
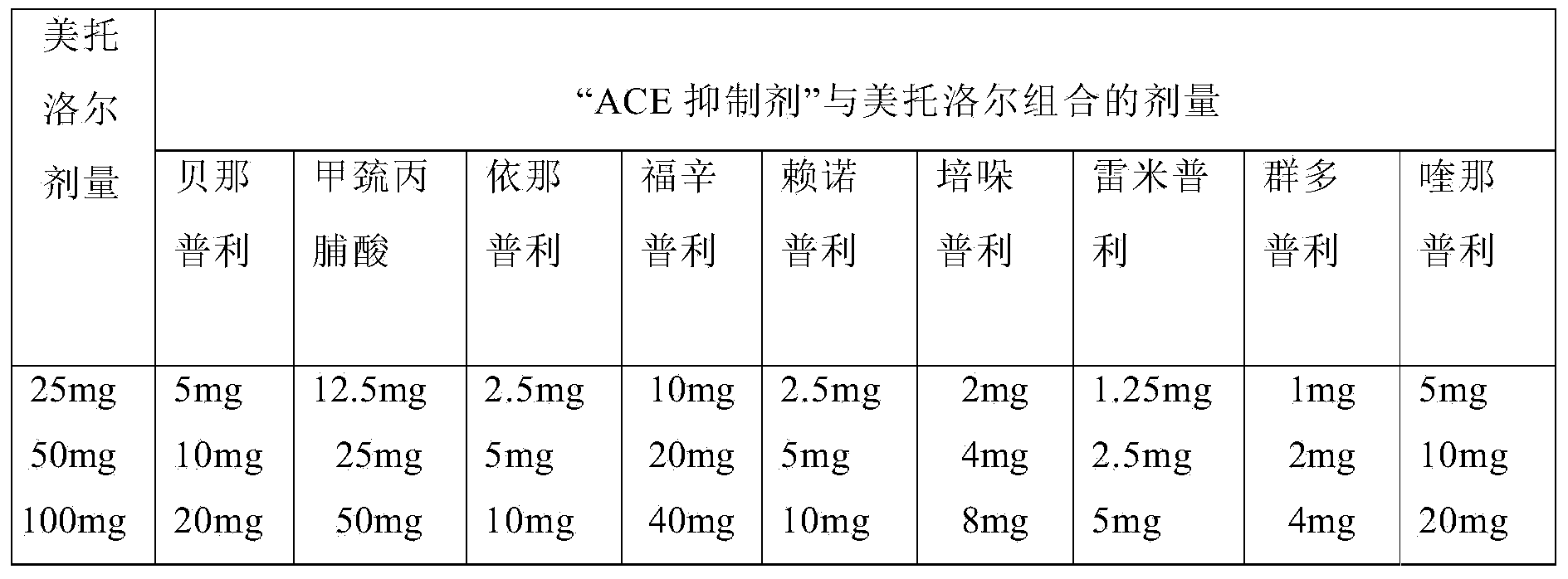
Methods for treating cardiovascular disorders
一种心血管、疾病的技术,应用在治疗心血管疾病领域,能够解决难以实现治疗性释放、高溶解性和高渗透性、难以配制缓释等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154]Embodiment 1: Metoprolol succinate ER / amlodipine besylate tablet
[0155] Table 1: Metoprolol succinate ER / Amlodipine besylate; Eq50mg tartrate / 10mg
[0156]
[0157]
[0158] method:
[0159] The microcrystalline cellulose spheres were provided with a capping layer I of ethyl cellulose. These block-coated pellets were subjected to metoprolol succinate layering with a binder in an aqueous solvent. Drug layered pills with Ethylcellulose and Opadry have ER Coating-I. Sustained release coating of Eudragit-II was provided using plasticizer, triethyl citrate and talc. The ER coated pellets were provided with a seal coat II followed by a PEG coat in a suitable solvent system. These PEG-coated pellets were mixed with Prosolv, amlodipine besylate, croscarmellose sodium, PEG and sodium stearyl fumarate and compressed into tablets. Coat the core tablet with Opadry.
[0160] Dissolution studies were performed on the tablets obtained from Example 1. The results of the...
Embodiment 2
[0163] Embodiment 2: Metoprolol succinate ER / amlodipine besylate tablet
[0164] Table 3: Metoprolol succinate ER / Amlodipine besylate; Eq25mg tartrate / 2.5mg
[0165]
[0166]
[0167] method:
[0168] The microcrystalline cellulose spheres were provided with a capping layer I of ethyl cellulose. These block-coated pellets were subjected to metoprolol succinate layering with a binder in an aqueous solvent system. Drug-layered pills with Ethylcellulose and Opadry had ER Coating-I. Sustained release coating-II of Eudragit was provided with plasticizer, triethyl citrate and talc. The ER coated pellets were provided with a seal coat II followed by a PEG coating in a suitable solvent system. These PEG coated pellets were mixed with Prosolv, croscarmellose sodium, PEG and sodium stearyl fumarate and compressed into tablets. Coat the core tablet with Opadry.
[0169] Dissolution studies were performed on the tablets obtained from Example 2. The results of the dissolutio...
Embodiment 3
[0173] Embodiment 3: Metoprolol succinate ER / amlodipine besylate tablet
[0174] Table 5: Metoprolol succinate ER / Amlodipine besylate; Eq25mg tartrate / 5m x
[0175]
[0176]
[0177] method:
[0178] The microcrystalline cellulose pellet spheroids were provided with an ethyl cellulose seal layer I. These block-coated pellets were subjected to metoprolol succinate layering with a binder in an aqueous solvent system. Drug-layered pills with Ethylcellulose and Opadry had ER Coating-I. Sustained release coating-II of Eudragit was provided with plasticizer, triethyl citrate and talc. The ER coated pellets were provided with a seal coat II followed by a PEG coating in a suitable solvent system. These PEG coated pellets were mixed with Prosolv, croscarmellose sodium, PEG and sodium stearyl fumarate and compressed into tablets. Using Opadry as a binder, the prepared metoprolol succinate core tablet was coated with amlodipine besylate. Prepared core tablets were coated w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com