A compound preparation used for treating diabetic painful sensory neuropathy and applications thereof
A compound preparation and neuropathy technology, applied in the field of medicine, can solve problems such as poor effect and side effects, and achieve the effects of good drug effect, no addiction and low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 compound preparation capsule preparation
[0054] Table 1-1
[0055]
[0056] Preparation process: prepare 1000 grains of product according to the above formula, the specific preparation method is as follows:
[0057] According to the usual capsule operation, take Anemarrhena lily extract powder, cilostazol, and methylcobalamin according to the formula in Table 1-1, dry granulate, pack into suitable capsules, and seal to obtain 1000 capsules.
Embodiment 2
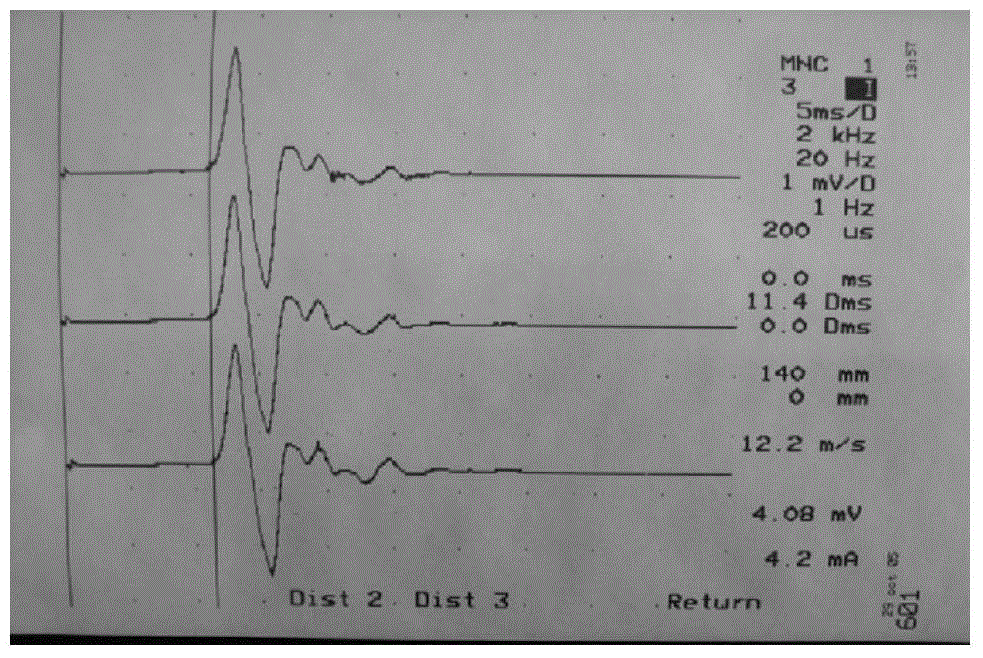
[0058] Embodiment 2 Improves the research of peripheral neuropathy in diabetic rats
[0059] 1. Research purpose: To observe the improving effect of the compound preparation capsule of the present invention on peripheral neuropathy in diabetic rats.
[0060] 2. Test drug: Prepared by Formula 1 of Example 1.
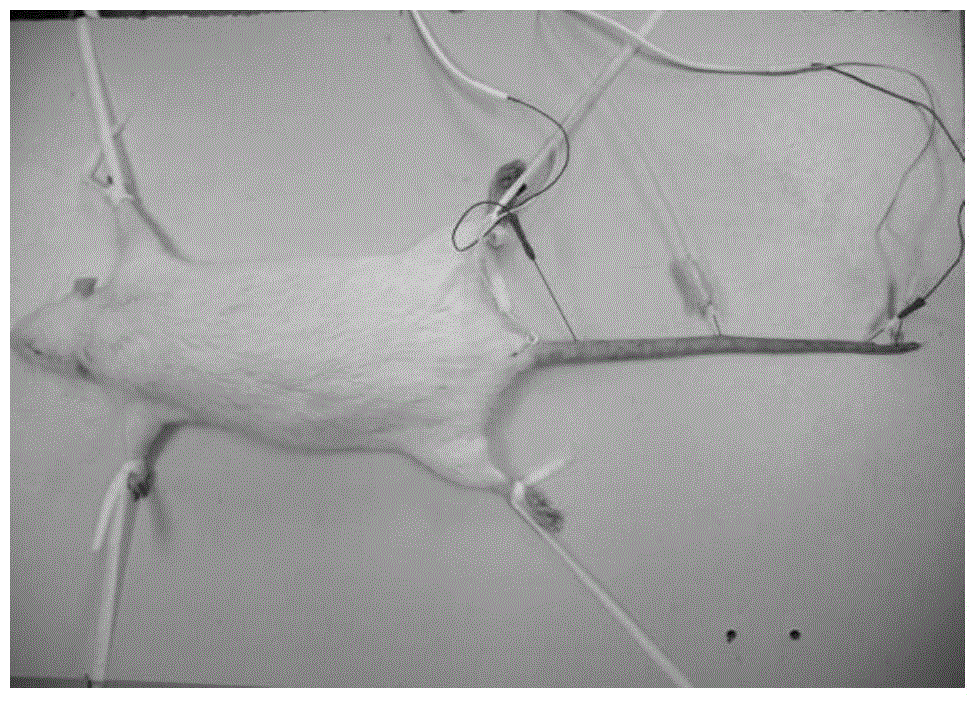
[0061] 3. Animal source, species, strain: Sprague-Dawley (SD) rats, body weight: 200-240g; sex: male; total number of animals: 55; purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences.
[0062] 4. Animal modeling and grouping: All SD rats were adaptively fed for 3 days, 8 SD rats were randomly selected as the normal control group (NC), and the remaining 47 SD rats were used for modeling. After fasting for 12 hours, STZ (streptozotocin) was dissolved in 0.1mol / L citric acid-sodium citrate buffer (pH4.2) to prepare a solution with a concentration of 1%, and a single dose of 60mg / kg By intraperitoneal injection, the normal control group (NC) was ...
Embodiment 3
[0097] Example 3 Study on Alleviating Effects on Diabetic Mice's Anxiety and Depression
[0098] 1. Research purpose: observe the alleviating effect of the compound preparation capsule of the present invention on the anxiety and depression of diabetic mice.
[0099] 2. Test drug: prepared by formula 1 in Example 1
[0100] 3. Animals: source, species, strain: clean grade male ICR mice, body weight: 18-20 g; sex: male; total number of animals: 120; purchased from Shanghai SIPPR-BK Experimental Animal Co., Ltd.
[0101] 4. Animal grouping and administration:
[0102] All mice were adaptively fed for 3 days, 12 mice were randomly selected as the normal control group (NC), and the rest were used for modeling. After fasting for 12 hours, STZ (streptozotocin) was dissolved in 0.1mol / L citric acid-sodium citrate buffer (pH4.2) to prepare a solution with a concentration of 1%, and a single dose of 60mg / kg By intraperitoneal injection, the normal control group (NC) was injected intr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com