Adsorbed acellular DTP-Sabin strain polio vaccine and preparation method thereof
A cell-free DPT and poliomyelitis technology, applied in the field of biomedicine, can solve the problems of frequent vaccinations and complicated vaccination procedures, and achieve the effects of reducing the number of vaccinations, simplifying the immunization procedures, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
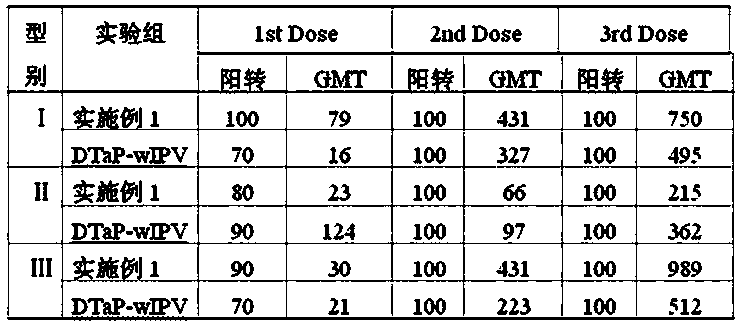
[0045] (1) According to the requirements of the 2010 edition of the Pharmacopoeia of the People's Republic of China, acellular pertussis antigens (PT, FHA, PRN), tetanus toxoid (TT) and diphtheria toxoid (DT), and Ⅰ, Ⅱ, and Ⅲ Sabin were prepared respectively. Strain poliovirus inactivation solution sIPV type Ⅰ, sIPV type Ⅱ, sIPV type Ⅲ ; Wherein the pertussis used for the preparation of pertussis toxin PT, filamentous hemagglutinin FHA and pertussis adhesin PRN Ⅰ The bacterial number of the corresponding CS bacterial strain is 58003-3; the bacterial number of Clostridium tetani used for the preparation of tetanus toxoid TT is 64008; the bacterial number of the diphtheria bacillus PW8 strain used for the preparation of diphtheria toxoid DT is 38007. From the serum laboratory of China National Institutes for Food and Drug Control; the polioviruses used to prepare type I, II and III poliovirus inactivation solutions of Sabin strains were respectively Ⅰ type is SabinSO+2, Ⅱ type...
Embodiment 2
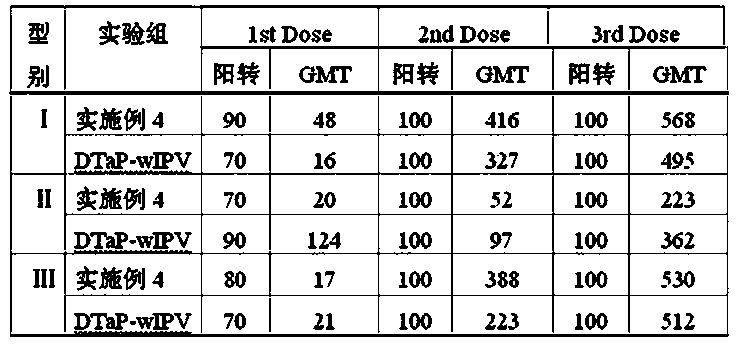
[0061] (1) According to the requirements of the 2010 edition of the Pharmacopoeia of the People's Republic of China, acellular pertussis antigens (PT, FHA, PRN), tetanus toxoid (TT) and diphtheria toxoid (DT), and Ⅰ, Ⅱ, and Ⅲ Sabin were prepared respectively. Strain poliovirus inactivation solution sIPV type Ⅰ, sIPV type Ⅱ, sIPV type Ⅲ ; Wherein the pertussis used for the preparation of pertussis toxin PT, filamentous hemagglutinin FHA and pertussis adhesin PRN Ⅰ The bacterial number of the corresponding CS bacterial strain is 58003-3; the bacterial number of Clostridium tetani used for the preparation of tetanus toxoid TT is 64008; the bacterial number of the diphtheria bacillus PW8 strain used for the preparation of diphtheria toxoid DT is 38007. From the serum laboratory of China National Institutes for Food and Drug Control; the polioviruses used to prepare type I, II and III poliovirus inactivation solutions of Sabin strains were respectively Ⅰ type is SabinSO+2, Ⅱ type...
Embodiment 3
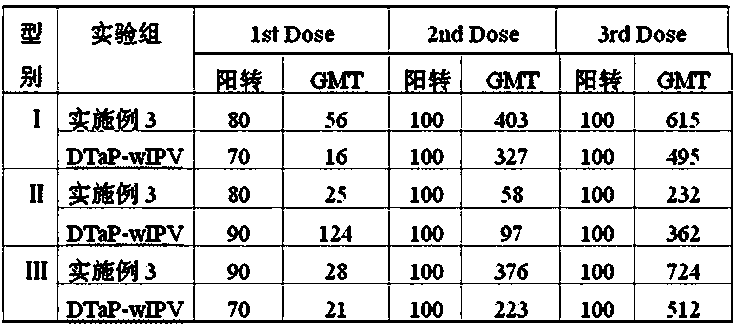
[0076] (1) According to the requirements of the 2010 edition of the Pharmacopoeia of the People's Republic of China, acellular pertussis antigens (PT, FHA, PRN), tetanus toxoid (TT) and diphtheria toxoid (DT), and Ⅰ, Ⅱ, and Ⅲ Sabin were prepared respectively. Strain poliovirus inactivation solution sIPV type Ⅰ, sIPV type Ⅱ, sIPV type Ⅲ ; Wherein the pertussis used for the preparation of pertussis toxin PT, filamentous hemagglutinin FHA and pertussis adhesin PRN ⅠThe bacterial number of the corresponding CS bacterial strain is 58003-3; the bacterial number of Clostridium tetani used for the preparation of tetanus toxoid TT is 64008; the bacterial number of the diphtheria bacillus PW8 strain used for the preparation of diphtheria toxoid DT is 38007. From the serum laboratory of China National Institutes for Food and Drug Control; the polioviruses used to prepare type I, II and III poliovirus inactivation solutions of Sabin strains were respectively Ⅰ type is SabinSO+2, Ⅱ type ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com