Co-precipitation synthesis method for Na2/3Ni1/3Mn2/3O2 as electrode material and preparation method of Na2/3Ni1/3Mn2/3O2 electrode
A technology of sodium nickel manganate and synthesis method, which is applied in the direction of manganate/permanganate, battery electrodes, nickel compounds, etc., can solve the problems of high cost, toxicity, poor safety, etc., and achieve low impedance, low cost, The effect of a small potential drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) NiSO 4 , MnSO 4 According to the nickel-manganese ratio of 0.8:1, the solutions are mixed with each other and stirred vigorously, and at the same time, the complexing agent concentrated ammonia water is added for complexation until the pH value is 8.5;
[0032] (2) Add 2mol / L NaOH solution to adjust the pH value to 11 for precipitation, and the obtained precipitate is filtered, washed and dried;
[0033] (3) Combine the dried product with the stoichiometric ratio of Na 2 CO 3 Perform ball milling and mixing for 5 hours;
[0034] (4) Put the ball-milled mixture into a muffle furnace for pretreatment at 400°C for 6h and high-temperature calcination at 800°C for 24h to obtain the final product.
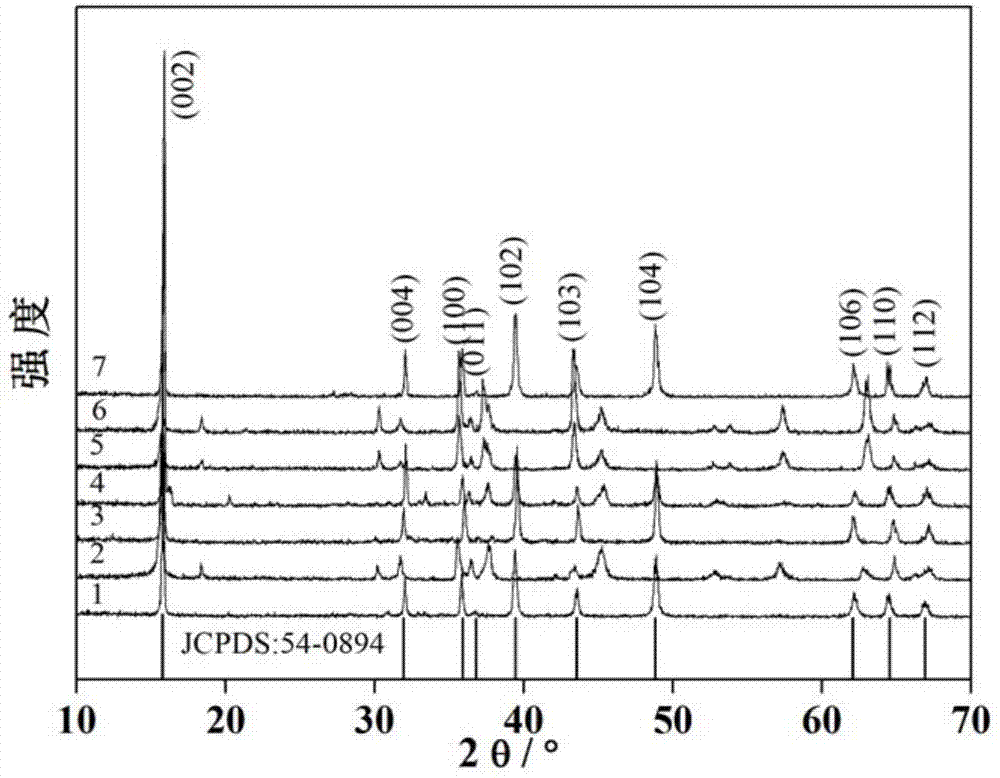
[0035] It can be seen from the XRD diagram that curve 1 corresponds to the standard card one by one, the characteristic peaks are sharp, and there are no obvious impurity peaks, indicating that Na 2 / 3 Ni 1 / 3 mn 2 / 3 o 2 The crystallinity is intact and the purity is high...
Embodiment 2
[0037] Example 1 (3) was changed to ball milling and mixing the dried product with stoichiometric NaOH for 5 hours. The rest of the synthesis conditions remain unchanged.
Embodiment 3
[0039] Example 1 (4) was changed to put the ball-milled mixture into a muffle furnace for pretreatment at 400° C. for 6 hours and high-temperature calcination at 750° C. for 24 hours to obtain the final product. The rest of the synthesis conditions remain unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com