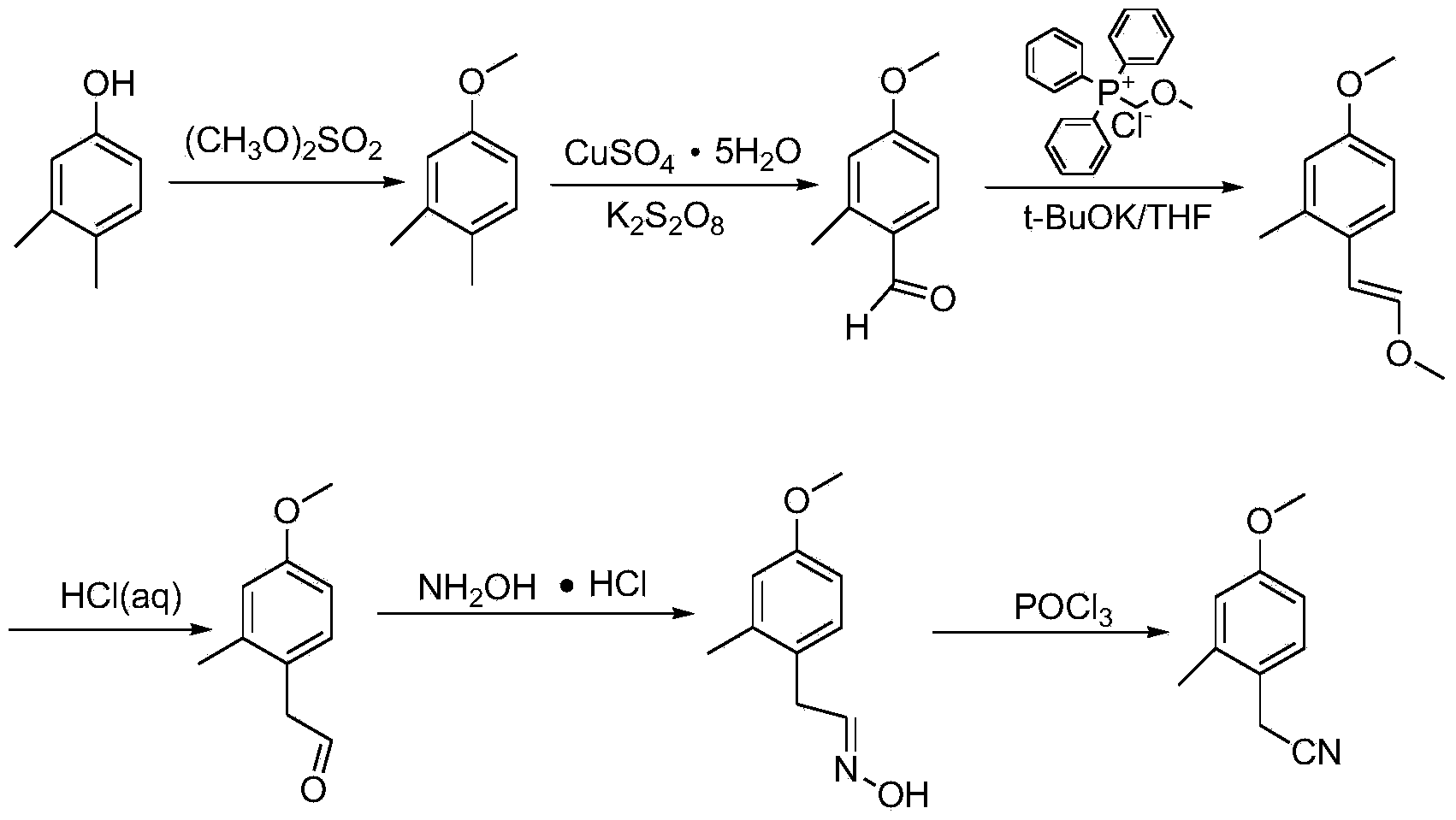
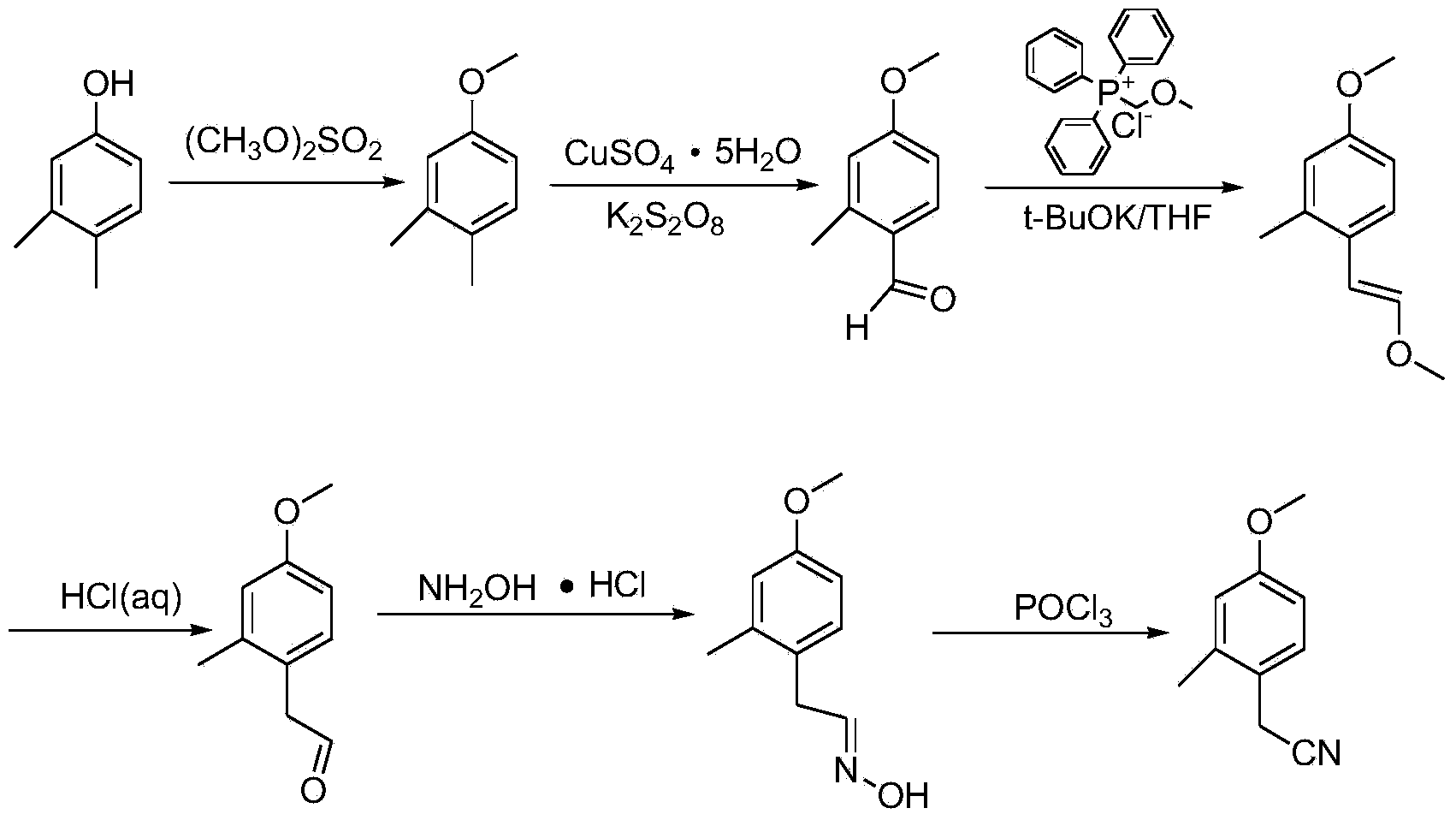
Synthesis method for 4-methoxy-2-methyl benzyl cyanide
A technology of methylbenzene acetonitrile and synthesis method, applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc., can solve the problems of low yield, difficult operation, difficult purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 100g of 3,4-dimethylphenol into a 500mL three-neck flask containing 123.9g of dimethyl sulfate and stir, start to add 131.5g of 32.3% NaOH solution dropwise, and control the temperature to 30°C during the dropwise addition. After the dropwise addition was completed, the temperature was controlled to 0-50°C and the reaction was stirred for 2 hours. Then, 500 mL of water was added, 500 mL×3 times of petroleum ether extraction, and the petroleum ether was concentrated to obtain 107 g of yellow liquid 3,4-dimethylanisole ( Yield 96%, GC=99%).
Embodiment 2
[0035] Add 50g of 3,4-dimethylanisole, 91.7g of copper sulfate pentahydrate and 297.7g of potassium persulfate into 1275mL of acetonitrile and 1275mL of water, heat up and control the temperature to 90°C, stir and react for half an hour, then cool down to 20°C to 30°C, extracted 500mL×3 times with dichloromethane, dried over anhydrous magnesium sulfate, concentrated dichloromethane to obtain 55.1g of 4-methoxy-2methylbenzaldehyde (100% yield, GC = 95%).
Embodiment 3
[0037] Add 110.89g of potassium tert-butoxide to a 2L three-neck flask containing 339g of chloromethyl ether triphenylphosphine salt and 660mL of THF (tetrahydrofuran). -2 methyl benzaldehyde dubbed solution. After the dropwise addition is completed, control the temperature to 10°C and react for 1 hour, extract 550mL with toluene×1 time, add 225mL ethanol and 225mL water to wash, dry over anhydrous magnesium sulfate, and concentrate to obtain high-purity 1-methoxy-2- 115.4 g of methyl-4-(2-methoxyvinyl)benzene (88.4% yield, GC=98%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com