Method for enhancing nonconformable gene expression in human cells
A technology for human embryonic kidney cells and cells, which can be used in DNA/RNA fragments, introduction of foreign genetic material using vectors, recombinant DNA technology, etc., and can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The preparation of embodiment 1, EBNA-D500mRNA
[0121] 1. Preparation of templates for in vitro transcription
[0122] The pRN3P-EBNA-D500 vector was linearized with restriction endonuclease SfiI, and the digested product was purified with a DNA purification and recovery kit, and used as a template for in vitro transcription of EBNA-D500, which can be stored at -80°C for future use.
[0123] 2. In vitro transcription of EBNA-D500mRNA
[0124] Utilize mMESSAGE In the T3 mRNA in vitro transcription kit, each component was added as shown in Table 1, reacted at 37° C. for 2 hours, and performed an in vitro transcription reaction to obtain EBNA-D500 mRNA (SEQ ID No.2).
[0125] Table 1 In vitro transcription system
[0126]
[0127] 3. Add 1 μL TURBO DNase to the in vitro transcription system, react at 37°C for 20 minutes, and digest the DNA template.
[0128] 4. Using Microspin TM S-200HR column (purchased from GE, Cat. No. 27-5120-01), the mRNA product obtained i...
Embodiment 2
[0135] Example 2. Chemical co-transfection of EBNA-D500mRNA and Episomal plasmid in human embryonic kidney cell line 293FT
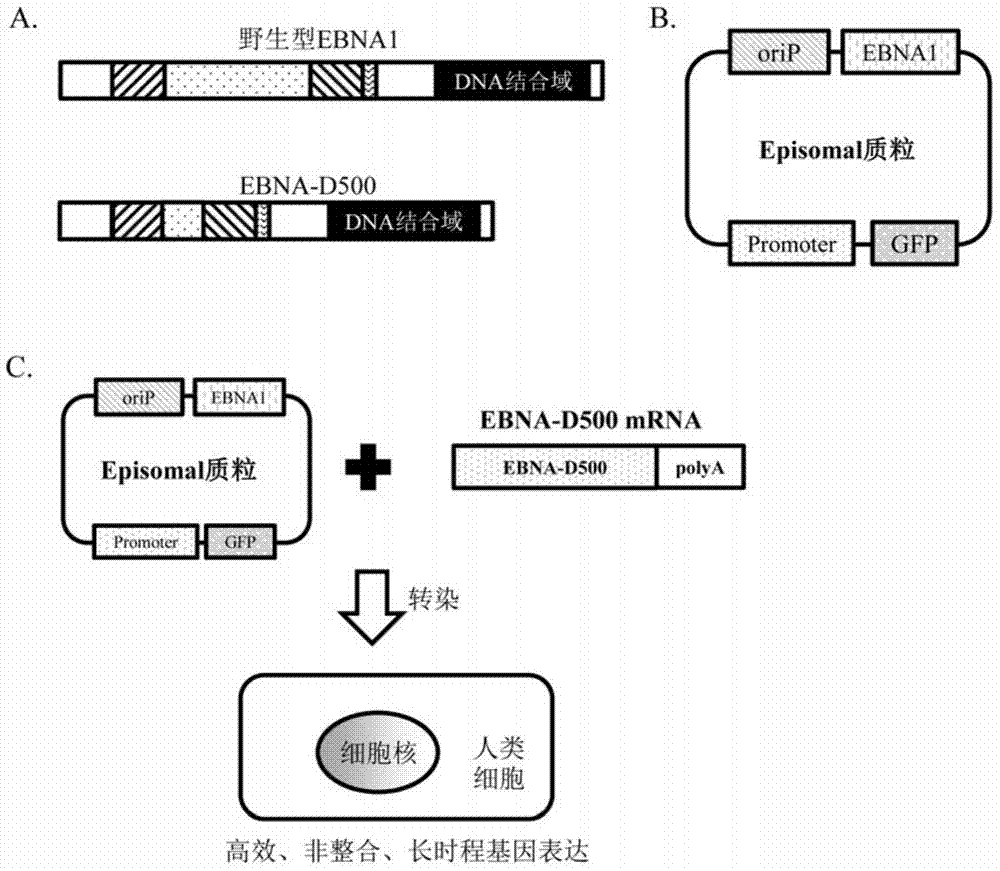
[0136] The Chinese name of Episomal plasmid is "non-integrated episomal plasmid", that is, a plasmid containing OriP / EBNA sequence, wherein OriP is in cis structure and EBNA is in trans structure, and can be used as an expression vector for any mammalian cell.
[0137] Episomal plasmid and EBNA-D500 mutant system such as figure 1 shown.
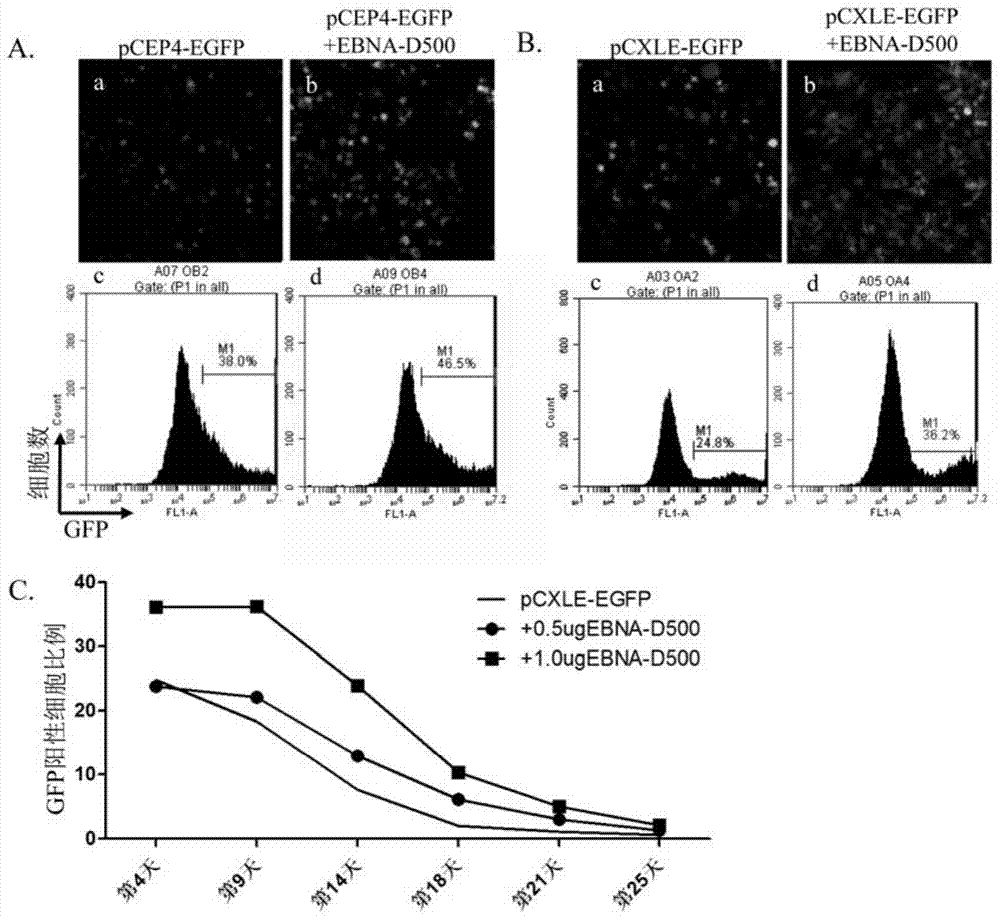
[0138] pCEP4-EGFP and pCXLE-EGFP are two different Episomal plasmids.
[0139] Specific steps are as follows:
[0140] 1. Passage human embryonic kidney cells 293FT to a 24-well plate one day before transfection, and the cell confluence is 40-50%.
[0141] 2. Replace the fresh 293FT cell culture medium two hours before transfection.
[0142] 3. According to Lipofectamine TM 2000 instructions for transfection. The mass ratio of liposomes to plasmids was 3:1. In the experimental group, 0.5μg EBNA-D500mRNA and 0.8μg...
Embodiment 3
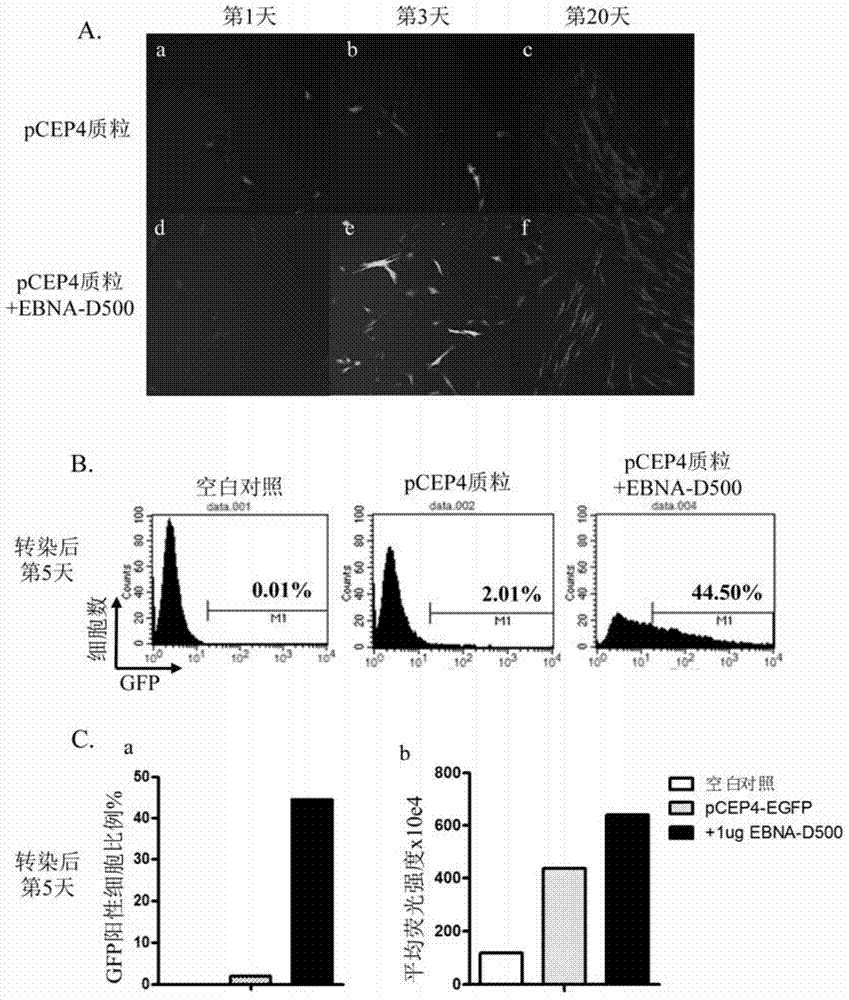
[0153] Example 3, Electrotransfection of EBNA-D500mRNA and Episomal plasmids in human fibroblasts
[0154] 1. Subculture human fibroblasts the day before transfection.
[0155] 2. Using Invitrogen Electrotransfection instrument, set the electrotransfection parameters before transfection, the parameters are 1550 volts, 10 milliseconds, 3 times.
[0156] 3. Dissociate human fibroblasts into single cells with 0.25% trypsin / EDTA, centrifuge to pellet the cells, discard the culture medium, resuspend the cells in PBS, and calculate the total number of cells.
[0157] 4. Add an appropriate volume of electrotransfer fluid Buffer R (Neon TM Electroporation kit comes with) to resuspend cells, adjust the cell concentration to 1x107 / mL, add 1 μg EBNA-D500mRNA and 0.5 μg Episomal plasmid (pCEP4-EGFP) to 10 μL of cells or add 0.5 μg Episomal plasmid (pCEP4-EGFP ), and the group without any plasmid was used as the blank control group.
[0158] Five, according to Neon TM Instructions fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com