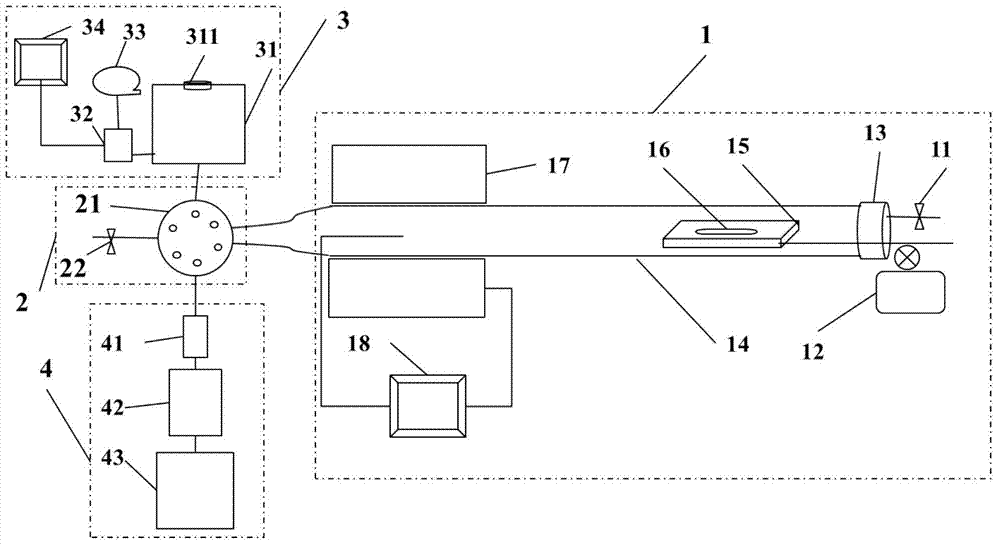
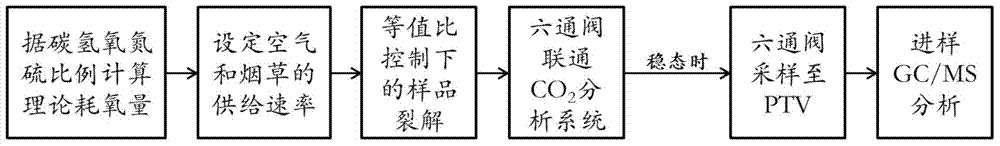
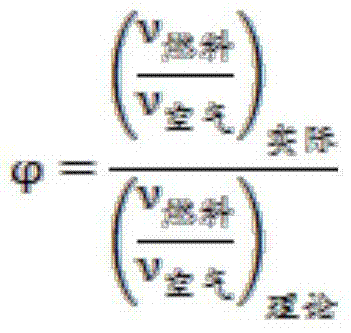Analytical method and analytical device for simulating cigarette burning and smoking based on controllable equivalence ratio method
A technology of analysis device and analysis method, applied in the direction of measuring device, analysis material, material separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: tobacco sample A is as shown in table 2 through elemental analysis result, and the mass summation of these five kinds of elements of carbon, hydrogen, oxygen, nitrogen, sulfur is 92.314%, after calculation, this tobacco sample of 1g burns fully (carbon is converted into CO 2 , the conversion of hydrogen to H 2 O, nitrogen converted to NO 2 , sulfur is converted to SO 2 ) after the oxygen consumption is 0.03969 mol, which is converted into a volume of 0.9713 liters under ideal conditions (temperature is 25 degrees and pressure is 1 atmosphere), that is, the amount of air consumed when 1 g of the tobacco sample is fully burned is 4.6252 liters. Taking the equivalence ratio as 1.5, we can get
[0053]
[0054] Take 20g of tobacco sample 16 and evenly spread it on an 80cm quartz boat. The advancing speed of the quartz boat is 4cm / min, then the fuel supply speed is 20g÷80cm×4cm / min=1g / min, and the air flow rate is 1g / min÷0.3243g / l=3.08 liters / minute.
...
Embodiment 2
[0056] Embodiment 2: The results of elemental analysis of tobacco sample B are as shown in Table 2. The total sum of these five elements of carbon, hydrogen, oxygen, nitrogen and sulfur is 95.211%. After calculation, 1g of this tobacco sample is fully combusted (carbon conversion for CO 2 , the conversion of hydrogen to H 2 O, nitrogen converted to NO2, sulfur converted to SO2), the oxygen consumption is 0.04203 mol, which is converted into a volume of 1.0285 liters in an ideal state (temperature is 25 ° C, pressure is one atmosphere), that is, 1 g of the tobacco sample is fully The amount of air consumed during combustion is 4.8976 liters. Taking the equivalence ratio as 1.1, we can get
[0057]
[0058] Take 10g of tobacco sample and evenly spread it on an 80cm quartz boat. The advancing speed of the quartz boat is 5cm / min, then the fuel supply speed is 10g÷80cm×5cm / min=0.625g / min, and the air flow rate is 0.625 g / min÷0.2246g / l=2.78 liters / minute.
[0059] Connect the...
Embodiment 3
[0060] Embodiment 3: The elemental analysis results of tobacco sample C are as shown in Table 2. The total sum of these five elements of carbon, hydrogen, oxygen, nitrogen and sulfur is 94.091%. After calculation, 1g of this tobacco sample is fully combusted (carbon conversion for CO 2 , the conversion of hydrogen to H 2 O, nitrogen converted to NO 2 , sulfur is converted to SO 2 ) after the oxygen consumption is 0.04333mol, which is converted into a volume of 1.0604 liters under ideal conditions (temperature is 25°C and pressure is 1 atmosphere), that is, the amount of air consumed when 1g of the tobacco sample is fully burned is 5.0495 liters. Taking the equivalence ratio as 3, we can get
[0061]
[0062] Take 30g of tobacco sample and spread it evenly on the 80cm quartz boat, the speed of the quartz boat is 3cm / min, then the fuel supply speed is 30g÷80cm×3cm / min=1.125g / min, then the air flow rate is 1.125 g / min÷0.5941g / l=1.89 liters / minute.
[0063] Connect the six...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com