Synthesis method of 4-substituted piperidine derivatives
A synthesis method and technology of derivatives, applied in the direction of organic chemistry and the like, can solve the problems of poor stability of intermediates, expensive sulfonylation reagent-trifluoromethanesulfonic anhydride, etc., and achieve low synthesis cost, improved synthesis efficiency, and suitable wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
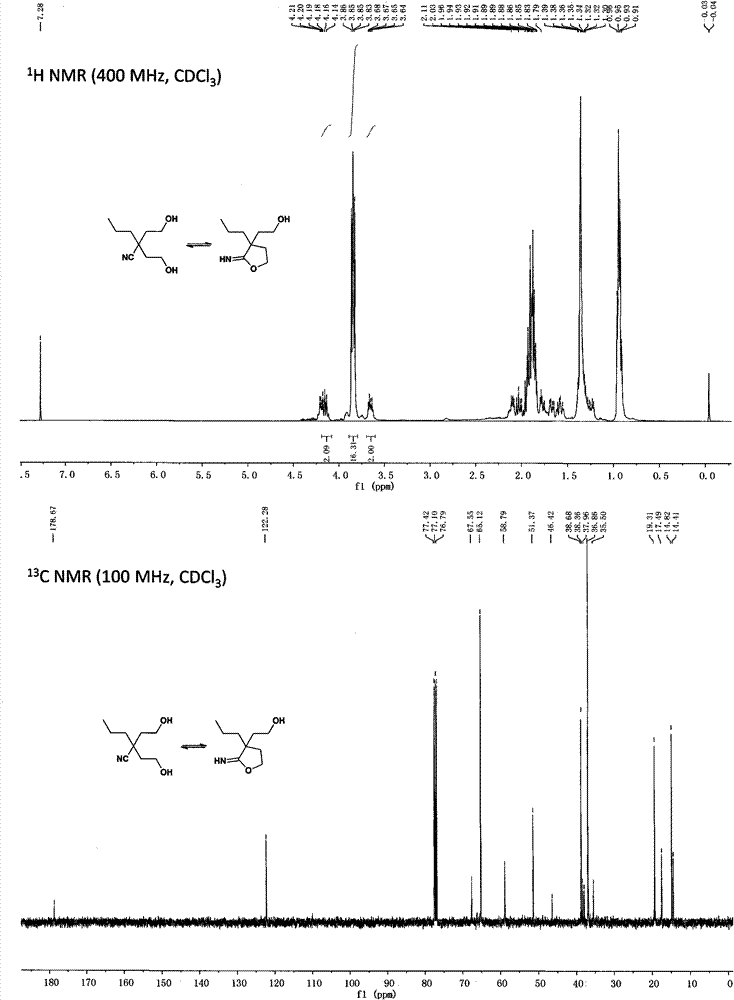
[0062] Preparation of α, α-bis(2-hydroxyethyl)valeronitrile and 2-(2-hydroxyethyl)-2-propyliminobutyrolactone
[0063]
[0064] Dissolve diisopropylamine (16.80mL, 120mmol) in 200mL of anhydrous tetrahydrofuran, lower the temperature to -30°C under the protection of argon, add 2.4mol / L n-butyl lithium (50mL, 120mmol) dropwise, and react for 0.5h; Add valeronitrile (4.16g, 50mmol) and continue stirring for 0.5h; add ethylene oxide (5.28g, 120mmol) dropwise and react for 0.5h; slowly rise to 0°C for 1h, add 5mL of water to terminate the reaction; distill THF off , adding 200mL dichloromethane and 50mL water for extraction, the aqueous phase was extracted again with 50mL dichloromethane; the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and distilled under reduced pressure to obtain a white solid with a yield of 58% .
[0065] NMR data of α,α-bis(2-hydroxyethyl)valeronitrile:
[0066] 1 H NMR (400MHz, ...
Embodiment 2
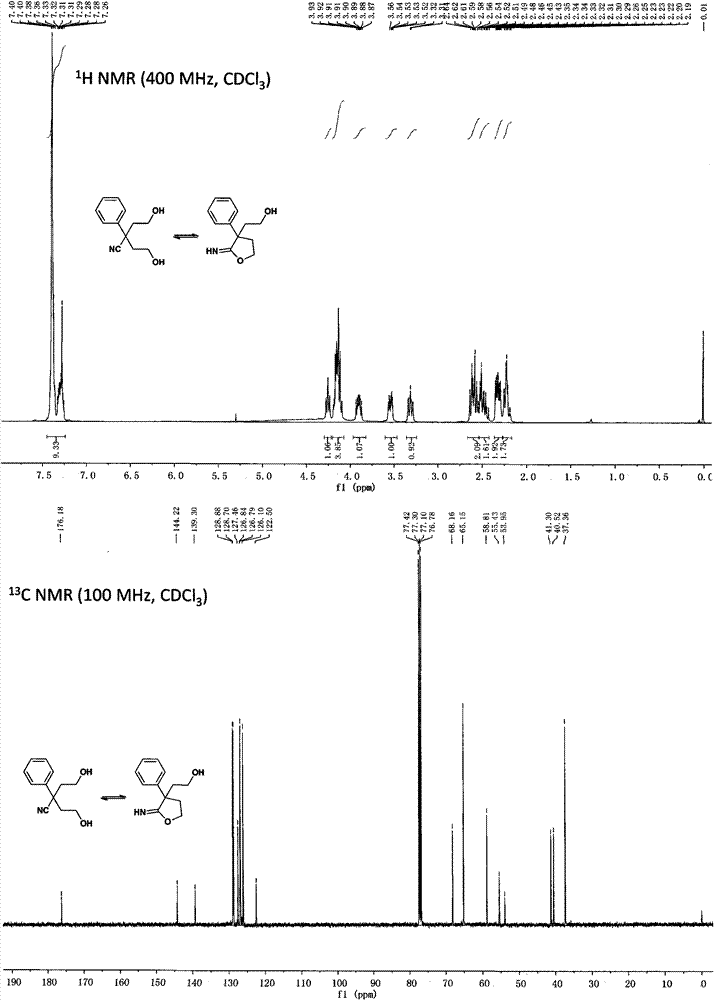
[0074] Preparation of α, α-bis(2-hydroxyethyl)phenylacetonitrile and 2-phenyl-2-(2-hydroxyethyl)iminobutyrolactone
[0075]
[0076] Dissolve diisopropylamine (7.70mL, 55mmol) in 50mL of anhydrous tetrahydrofuran, cool down to -30°C under argon protection, drop in 2.4mol / L n-butyllithium (23mL, 55mmol), and react for 0.5h; Add phenylacetonitrile (2.92g, 25mmol), continue to stir for 0.5h; add dropwise ethylene oxide (2.42g, 55mmol), react for 0.5h; slowly rise to 0°C for 1h, add 5mL of water to terminate the reaction; evaporate tetrahydrofuran , adding 100mL dichloromethane and 50mL water for extraction, the aqueous phase was extracted again with 50mL dichloromethane; the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and separated by silica gel column chromatography to obtain a white solid, the yield 68%.
[0077] NMR data of α,α-bis(2-hydroxyethyl)phenylacetonitrile:
[0078] 1 H NMR (400MHz, CDCl ...
Embodiment 3
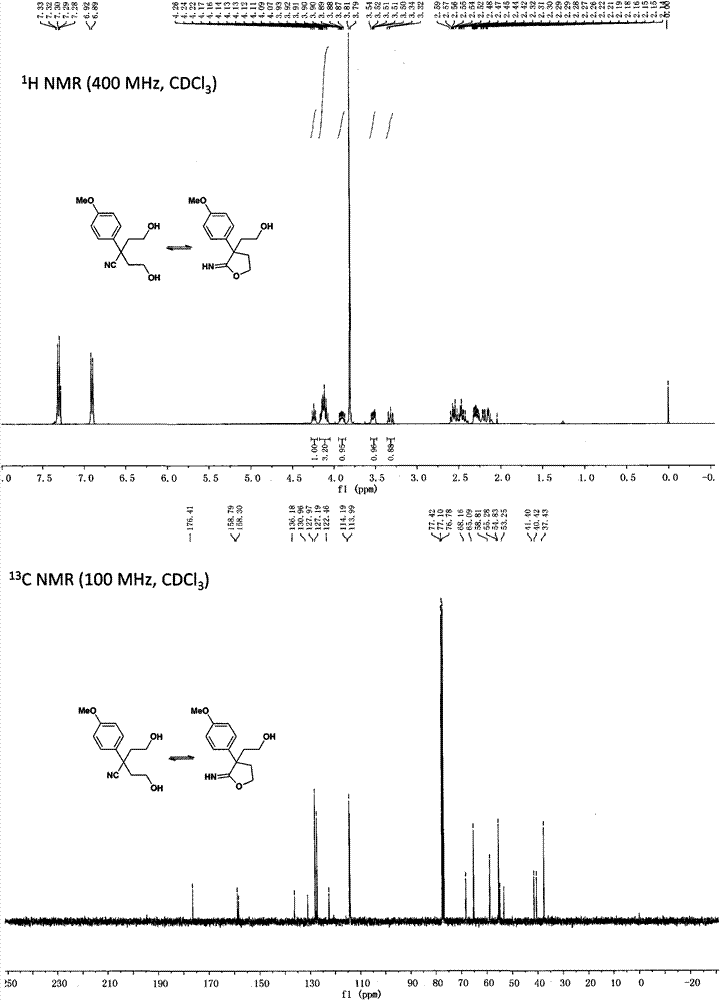
[0086] Preparation of α, α-bis(2-hydroxyethyl)-p-methoxyphenylacetonitrile and 2-p-methoxyphenyl-2-(2-hydroxyethyl)iminobutyrolactone
[0087]
[0088] Dissolve diisopropylamine (3.30mL, 24mmol) in 20mL of anhydrous tetrahydrofuran, lower the temperature to -30°C under the protection of argon, add 2.4mol / L n-butyllithium (10mL, 24mmol) dropwise, and react for 0.5h; Add p-methoxyphenylacetonitrile (1.47g, 10mmol), continue to stir for 0.5h; drop in ethylene oxide (1.06g, 24mmol), react for 0.5h; slowly rise to 0°C for 1h, add 5mL of water to terminate the reaction ; Evaporate tetrahydrofuran, add 50mL dichloromethane and 20mL water for extraction, the water phase is extracted again with 50mL dichloromethane; combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate and separate by silica gel column chromatography to obtain white Solid, yield 66%.
[0089] NMR data of α,α-bis(2-hydroxyethyl)-p-methoxyphenylacetonitrile:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com