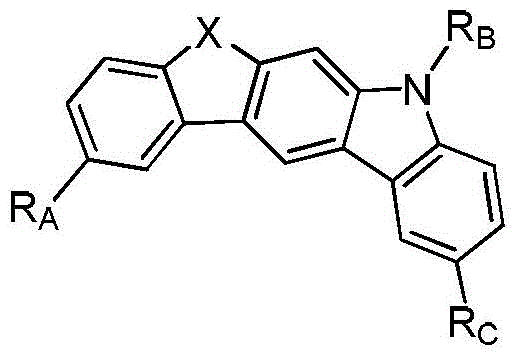
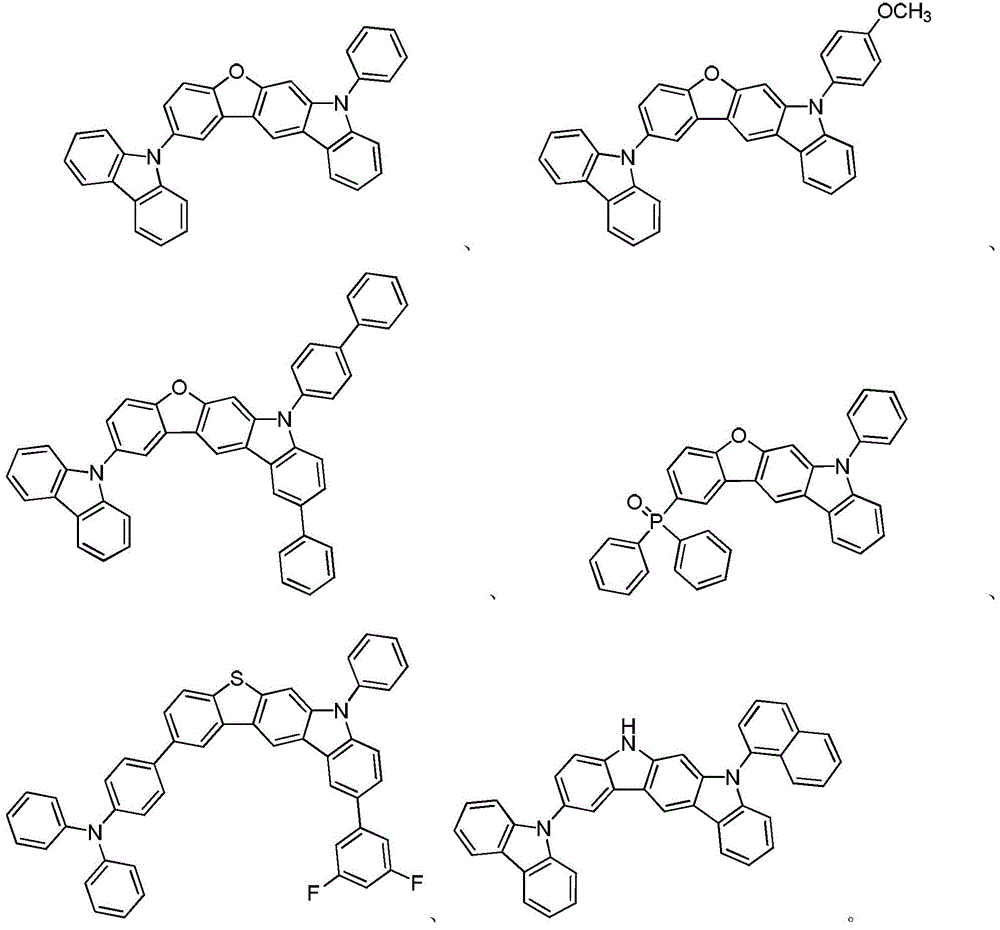
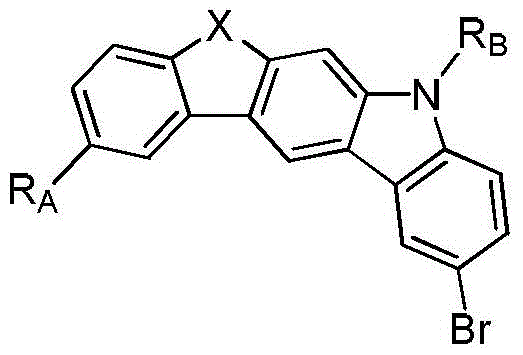
Compounds containing heteroatom bridging carbazole structural units and their preparation methods and applications
A compound and hydrogen atom technology, applied in the field of compounds containing heteroatom bridging carbazole structural units and their preparation, can solve the problems of practical application and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0158] According to the preparation method of the starting reactant 3f in Example 3, only replacing 3e with 5e, the target compound 5f was obtained. Yield: 71%.
[0159] The starting reactant 6d used in the following embodiment 6 is prepared according to the following method:
[0160] step 1: Synthesis
[0161]
[0162] According to step 1 of the aforementioned method for preparing 1d, 2,8-dibromooxyfluorene was replaced by 2,8-dibromocarbazole to obtain the target compound 6a. Yield: 46%.
[0163] Step 2: Synthesis
[0164]
[0165] According to step 2 of the aforementioned method for preparing 1d, 1a was replaced by 6a to obtain the target compound 6b. Yield: 72%.
[0166] Step 3: Synthesis
[0167]
[0168] According to step 3 of the aforementioned method for preparing 1d, 1b was replaced with 6b to obtain the target compound 6c. Yield: 85%.
[0169] Step 4: Synthesis
[0170]
[0171] According to step 4 of the aforementioned method for preparing...
Embodiment 1
[0172] Embodiment 1, compound preparation of
[0173]
[0174] Into a 100mL reaction flask, put 7.2mmol of compound 1d, 8.6mmol of bromobenzene, 0.2mmol of CuI, and 22.0mmol of K 2 CO 3 and 20 mL of xylene, and then heated to 140° C. for reflux reaction for 6 hours. Add 30 mL of ethyl acetate and 30 mL of water to the system, stir and separate the liquids, extract the aqueous phase twice with 30 mL of ethyl acetate, wash the organic phase twice with 30 mL of saturated brine, combine the organic phases, and spin dry to obtain a yellow solid. After passing through a silica gel column and eluting with petroleum ether:dichloromethane=10:1, a white solid product was obtained, with a yield of 65%.
[0175] 1 HNMR (CDCl 3 ,300MHz):δ=8.55-8.57(d,1H),8.09-8.12(d,2H),7.94-7.96(d,1H),7.80(s,1H),7.40-7.63(m,13H),7.25 -7.29(m,4H);
[0176] Glass transition temperature Tg: 266°C;
[0177] UV absorption wavelength: 275nm, 295nm;
[0178] Fluorescence emission wavelength: 448nm. ...
Embodiment 2
[0181] Embodiment 2, compound Synthesis
[0182]
[0183] According to the steps of Example 1, only the bromobenzene was replaced by 4-methoxybromobenzene to obtain the target compound 2e with a yield of 82%.
[0184] 1 HNMR (CDCl 3 ,300MHz):δ=8.55-8.57(d,1H),8.10-8.12(d,2H),7.94-7.96(d,1H),7.63-7.66(m,3H),7.49-7.51(m,6H) ,7.41(s,1H),7.25-7.33(m,4H),6.97-6.99(d,2H),3.83(s.3H);
[0185] Glass transition temperature Tg: 289°C;
[0186] UV absorption wavelength: 285nm, 305nm, 315nm;
[0187] Fluorescence emission wavelength: 418nm.
[0188] As can be seen from the above, the structure of the white solid product is correct, and it is a compound shown in formula I
[0189]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com