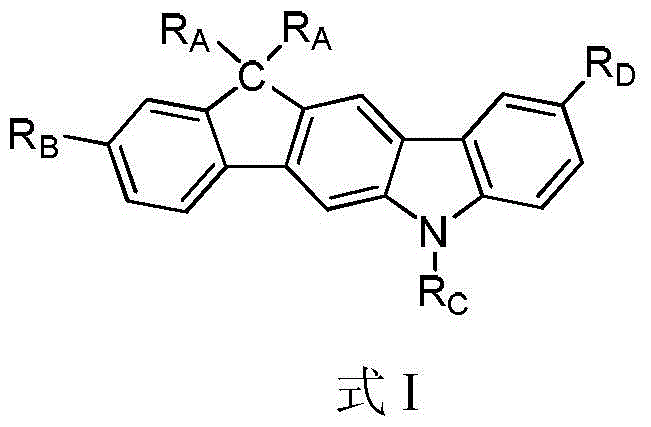
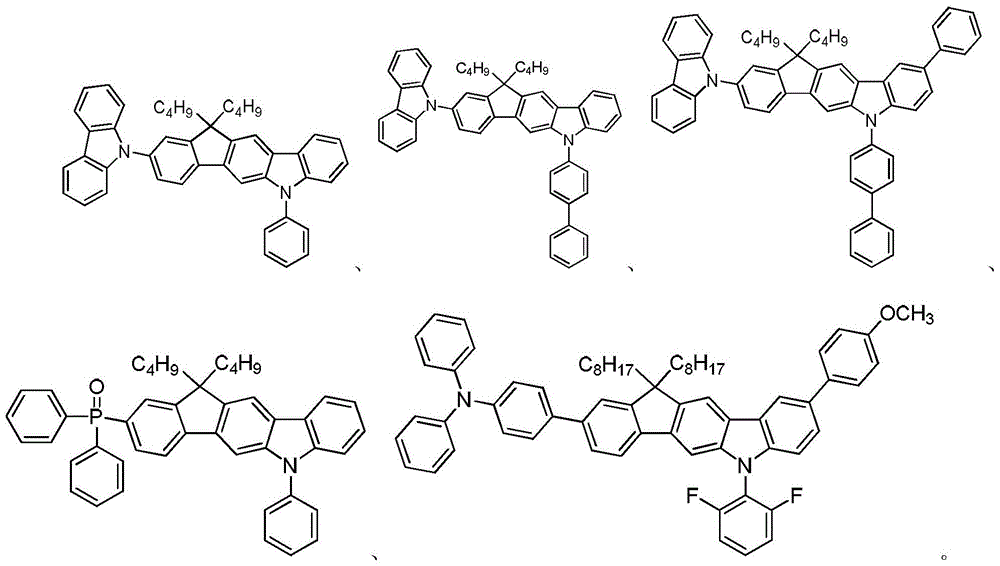
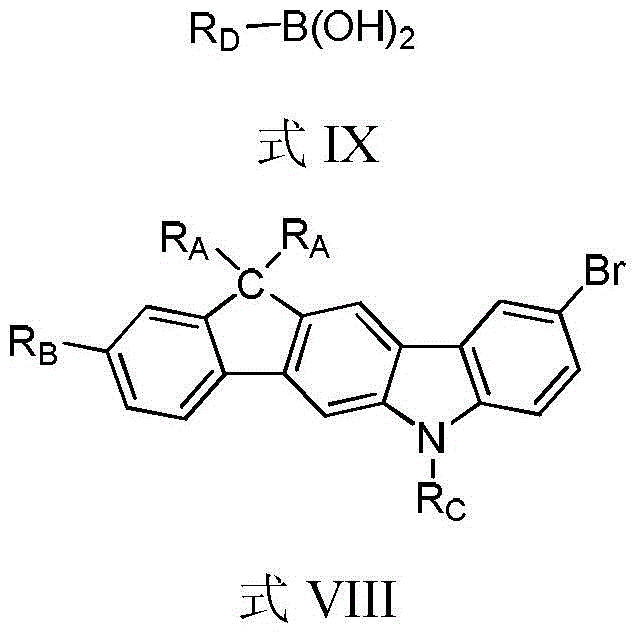
Compounds containing carbon-bridged carbazole structural units and their preparation methods and applications
A compound and organic technology, applied in the field of compounds containing carbon-bridged carbazole structural units and their preparation, can solve the problems such as the distance of practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Embodiment 1, compound preparation of
[0137]
[0138] Into the 100mL reaction flask, drop into the K 2 CO 3 and 20 mL of xylene, and then heated to 140° C. for reflux reaction for 6 hours. Add 30 mL of ethyl acetate and 30 mL of water to the system, stir and separate the liquids, extract the aqueous phase twice with 30 mL of ethyl acetate, wash the organic phase twice with 30 mL of saturated brine, combine the organic phases, and spin dry to obtain a yellow solid. After passing through a silica gel column and eluting with petroleum ether:dichloromethane=10:1, a white solid product was obtained, with a yield of 45%.
[0139] 1 HNMR (CDCl 3 ,300MHz):δ=8.85(s,1H),8.55-8.57(d,2H),8.09-8.12(m,3H),7.01-7.19(m,4H),7.94-7.96(d,1H),7.29 -7.45(m,15H),1.87-1.92(t,4H),1.24-1.29(m,8H),0.86-0.92(t,6H);
[0140] Glass transition temperature Tg: 287°C;
[0141] UV absorption wavelength: 255nm, 318nm;
[0142] Fluorescence emission wavelength: 454nm.
[0143] As can be s...
Embodiment 2
[0144] Embodiment 2, compound Synthesis
[0145] According to the steps of Example 1, only bromobenzene was replaced by 4-bromobiphenyl to obtain the target compound with a yield of 72%.
[0146] 1 HNMR (CDCl 3 ,300MHz):δ=8.87(s,1H),8.55-8.57(d,2H),8.09-8.12(m,3H),7.01-7.19(m,4H),7.92-7.94(d,1H),7.76 -7.79(d,2H),7.63-7.68(m,4H),7.29-7.45(m,13H),1.87-1.92(t,4H),1.24-1.29(m,8H),0.86-0.92(t, 6H).
[0147] Glass transition temperature Tg: 265°C;
[0148] UV absorption wavelength: 265nm, 315nm;
[0149] Fluorescence emission wavelength: 446nm.
[0150] As can be seen from the above, the structure of the white solid product is correct, and it is a compound shown in formula I
Embodiment 3
[0151] Embodiment 3, compound Synthesis
[0152]
[0153] In the 100mL reaction bottle, drop 0.74mmol of 3f, 0.81mmol of phenylboronic acid, 2.96mmol of sodium carbonate, 0.02mmol of Pd(PPh 3 ) 4 , and then put into 20mL of toluene, 5mL of ethanol and 5mL of water, and react at 100°C for 8 hours. Add 20 mL of saturated brine and 20 mL of ethyl acetate to the system, stir and separate the liquids, extract the aqueous phase twice with 20 mL of ethyl acetate, wash the organic phase twice with 20 mL of saturated brine, combine the organic phases, and spin dry to obtain a black solid . After passing through a silica gel column, washing with petroleum ether: dichloromethane=6:1 to obtain a white solid product, yield: 68%
[0154] 1 HNMR (CDCl 3 ,300MHz):δ=8.83(s,1H),8.51-8.53(d,2H),8.09-8.12(m,2H),7.01-7.94(m,26H),1.82-1.92(t,4H),1.24 -1.26(m,8H),0.88-0.92(t,6H).
[0155] Glass transition temperature Tg: 266°C;
[0156] UV absorption wavelength: 245nm, 315nm;
[0157] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com