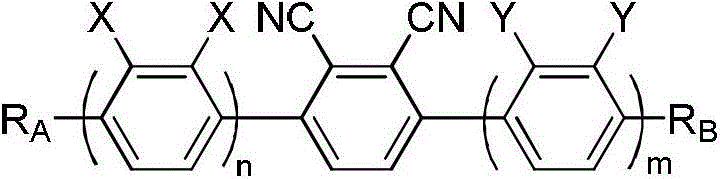
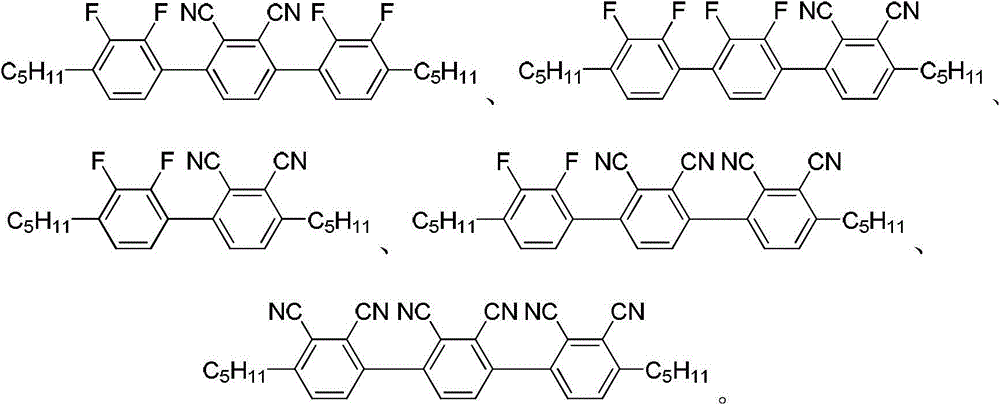
Poly-fluorine poly-cyanogen liquid crystal compound as well as preparation method and application thereof
A compound, dicyano technology, applied in the field of polyfluorine and polycyanide liquid crystal compounds and their preparation, can solve the problems of high viscosity, poor optical stability, low resistivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1, compound 2,3-dicyano-4-(2,3-difluoro-4-n-pentylphenyl)-2',3'-difluoro-4'-n-pentylbiphenyl preparation
[0074] step 1: Synthesis
[0075]
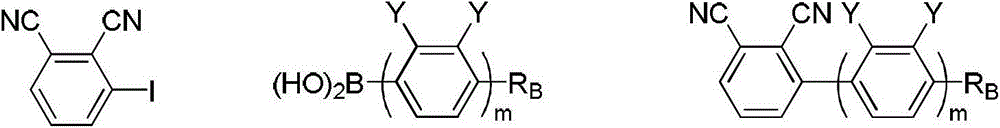
[0076] Put 0.10mol of 2,2,6,6-tetramethylpiperidinyllithium hexane solution into a 250mL reaction bottle, and put 0.05mol of ZnCl into the system at 0°C 2 • TMEDA (catalyst), then kept stirring at 0°C for 20 minutes. Add dropwise 0.05 mol of tetrahydrofuran solution of phthalonitrile represented by formula II to the system, and keep 0° C. for negative ionization reaction for 2 hours after the dropwise addition is completed. Then, 0.15 mol of iodine in tetrahydrofuran solution was added dropwise to the system. After the dropwise addition, iodination reaction was carried out for 1 hour and then reacted at room temperature for 2 hours. After the reaction was completed, 100 mL of saturated sodium thiosulfate solution was added to the system, and after stirring for 20 minutes, 40 mL of ethyl acetate was added, stirred ...
Embodiment 2
[0088] Example 2, compound 2,3-difluoro-4-(2,3-dicyano-4-n-pentyl)phenyl-2',3'-difluoro-4'-n-pentylbiphenyl synthesis
[0089] step 1: Synthesis
[0090] Prepared according to the same steps as in Example 1 step 1) Yield: 65%.
[0091] Step 2: Synthesis
[0092]
[0093] With reference to step 2 of Example 1, the 2,3-difluoro-4-n-pentylphenylboronic acid in step 2 of Example 1 was replaced with n-pentylphenylboronic acid to prepare the compound 1,2-dicyano- 3-n-Pentylbenzene, yield: 76%.
[0094] Step 3: Synthesis
[0095]
[0096] With reference to step 1 of Example 1, the phthalonitrile in step 1 of Example 1 is replaced by the 1,2-dicyano-3-n-pentylbenzene obtained in step 2) of this example, and other operations are carried out with In step 1 of Example 1, the compound 1,2-dicyano-3-iodo-6-n-pentylbenzene was prepared, and the yield was 68%.
[0097] Step 4: Synthesis
[0098] Referring to step 2 of Example 1, replace 1,2-dicyano-3-iodobenzene and 2,...
Embodiment 3
[0103] Embodiment 3, the synthesis of compound 2,3-dicyano-2',3'-difluoro-4,4'-di-n-pentylbiphenyl
[0104]
[0105] In this example, referring to Step 2 of Example 1, 1,2-dicyano-3-iodobenzene and 2,3-difluoro-4-n-pentyl-phenylboronic acid in Step 2 of Example 1 are replaced respectively 2', 3'-difluoro-4'-n-pentyl-2,3-dicyano-4-iodo-biphenyl, n-pentylphenylboronic acid prepared in step 3 of Example 1, other operations The target compound 2,3-dicyano-2',3'-difluoro-4,4'-di-n-pentylbiphenyl was prepared in the same way as in step 2 of Example 1, with a yield of 89%.
[0106] 1 H NMR (CDCl 3 , 300MHz): δ=7.64(s, 2H), 7.07-7.09(d, 2H), 2.91-2.96(t, 2H), 2.68-2.73(t, 2H), 1.36-1.40(m, 9H), 0.88 -0.92 (m, 9H).
[0107] Δn=0.0688, Δε=-2.8.
[0108] As can be seen from the above, the structure of the white solid product is correct, and it is the compound 2,3-dicyano-2',3'-difluoro-4,4'-di-n-pentylbiphenyl shown in formula I
[0109]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com