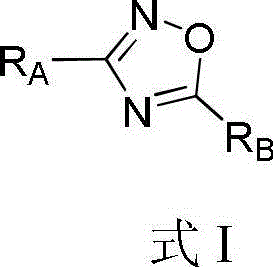
1,2,4-oxadiazole compound as well as preparation method and application thereof
A technology of compound, Z1-A1-Z2, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
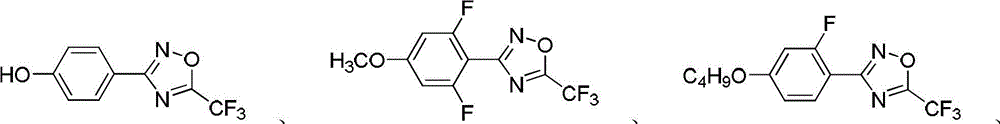
[0066] Embodiment 1, the preparation of compound 4-(5-trifluoromethyl-1,2,4-oxadiazolyl)phenol
[0067] step 1: Synthesis
[0068]
[0069] Into a 250mL reaction bottle, put 5.3mmol of 4-cyanophenol, 8.0mmol of hydroxylamine hydrochloride and 10.6mmol of sodium bicarbonate, put in 30mL of ethanol, and react under reflux at 80°C for 6h. Add 100 mL of water and 20 mL of ethyl acetate to the system, stir and separate the liquids, extract the aqueous phase twice with 30 mL of ethyl acetate, wash the organic phase twice with 30 mL of saturated brine, combine the organic phases, and spin dry to obtain a white solid. Yield: 95%.
[0070] Step 2: Synthesis
[0071]
[0072] Into a 250mL reaction flask, put 2.0mmol of 4-aminoximimophenol and 2.3mmol of trifluoroacetic anhydride in step 1, put in 50mL of toluene, and reflux at 110°C for ring closure reaction for 4h. The solvent was spin-dried, passed through a silica gel column, and washed with petroleum ether to obtain a...
Embodiment 2
[0076] Embodiment 2, the synthesis of compound 3-(2,6-difluoro-4-methoxyphenyl)-5-trifluoromethyl-1,2,4-oxadiazole
[0077] This embodiment refers to Example 1, and the 4-cyanophenol in step 1 is replaced by 2,6-difluoro-4-methoxybenzonitrile, and the 4-aminoximimophenol in step 2 is replaced by 2,6 -difluoro-4-methoxyaminoximinylbenzene, other operations are the same as in Example 1, and the target compound 3-(2,6-difluoro-4-methoxyphenyl)-5-trifluoromethyl- 1,2,4-oxadiazole.
[0078] step 1: Synthesis
[0079]
[0080] Yield: 90%.
[0081] Step 2: Synthesis
[0082]
[0083] Yield: 88%
[0084] The NMR data of this compound were measured: 1 H NMR (CDCl 3 , 300 MHz): δ = 6.53-6.56 (d, 2H), 3.82 (s, 3H). As can be seen from the above, the structure of the colorless liquid product is correct, and it is the compound 3-(2,6-difluoro-4-methoxyphenyl)-5-trifluoromethyl-1,2,4- Oxadiazole
[0085]
[0086] The liquid crystal performance data of this compound w...
Embodiment 3
[0087] Embodiment 3, the synthesis of compound 3-(4-butoxy-2-fluorophenyl)-5-trifluoromethyl-1,2,4-oxadiazole
[0088] This example refers to Example 1, and the 4-cyanophenol in step 1 is replaced by 2-fluoro-4-butoxybenzonitrile, and the 4-aminoximiphenol in step 2 is replaced by 2-fluoro-4- Butoxyaminoximine base benzene, other operation is the same as embodiment 1, prepares target compound 3-(4-butoxy group-2-fluorophenyl)-5-trifluoromethyl-1,2,4-oxadiazole .
[0089] step 1: Synthesis
[0090]
[0091] Yield: 94%.
[0092] Step 2: Synthesis
[0093]
[0094] Yield: 81%
[0095] The NMR data of this compound were measured: 1 H NMR (CDCl 3 , 300MHz): δ=8.05-8.10(m, 1H), 6.80-6.84(m, 2H), 4.04-4.06(t, 2H), 1.72-1.78(m, 2H), 1.42-1.45(m, 2H) , 0.88-091 (t, 3H). As can be seen from the above, the white solid product has a correct structure and is the compound 3-(4-butoxyl-2-fluorophenyl)-5-trifluoromethyl-1,2,4-oxadiazole shown in formula I
[0096]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com