Application of cystatin SN and AFP in preparation of marker for diagnosis and predicting of liver cancer
A technology for markers and liver cancer, applied in the field of medical detection, can solve the problem that the specificity and sensitivity of liver cancer cannot meet the monitoring of liver cancer, and achieve the effect of easy portability, good repeatability, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Establishment of Cystatin SN Serum Detection Reaction System and Its Optimization
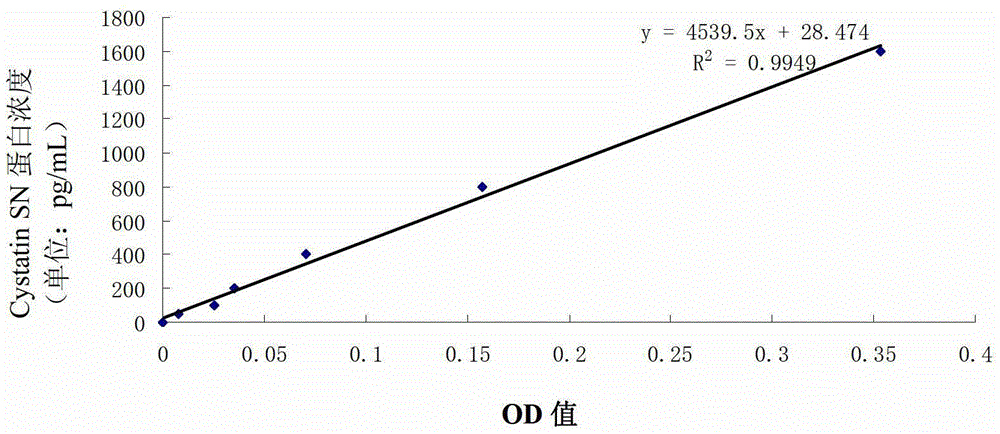
[0025] Coat the ELISA plate with a mouse anti-human Cystatin SN monoclonal antibody at a concentration of 5 μg / mL, coat it overnight at 4°C, and wash the plate; then block it in 2% BSA at room temperature for 2 hours, and wash the plate; Then concentration is 0pg / mL, 50pg / mL, 100pg / mL, 200pg / mL, 400pg / mL, 800pg / mL, the Cystatin SN protein standard substance of 1600pg / mL (the aminoacid sequence of coding Cystatin SN protein is as SEQ ID NO. 1) and serum samples were added to the blocked plate, reacted at 37°C for 1 hour, and washed the plate; then reacted with rabbit anti-human Cystatin SN polyclonal antibody with a concentration of 0.5 μg / mL HRP at 37°C for 1 hour, Wash the plate; react with tetramethylbenzidine (TMB) for 2-3 minutes, and finally stop the reaction with 2M sulfuric acid, and detect the OD value at 450nm ( figure 1 ). Depend on figure 1 It can be seen that the...
Embodiment 2
[0032]Embodiment 2 Cystatin SN enzyme-linked immunoassay kit
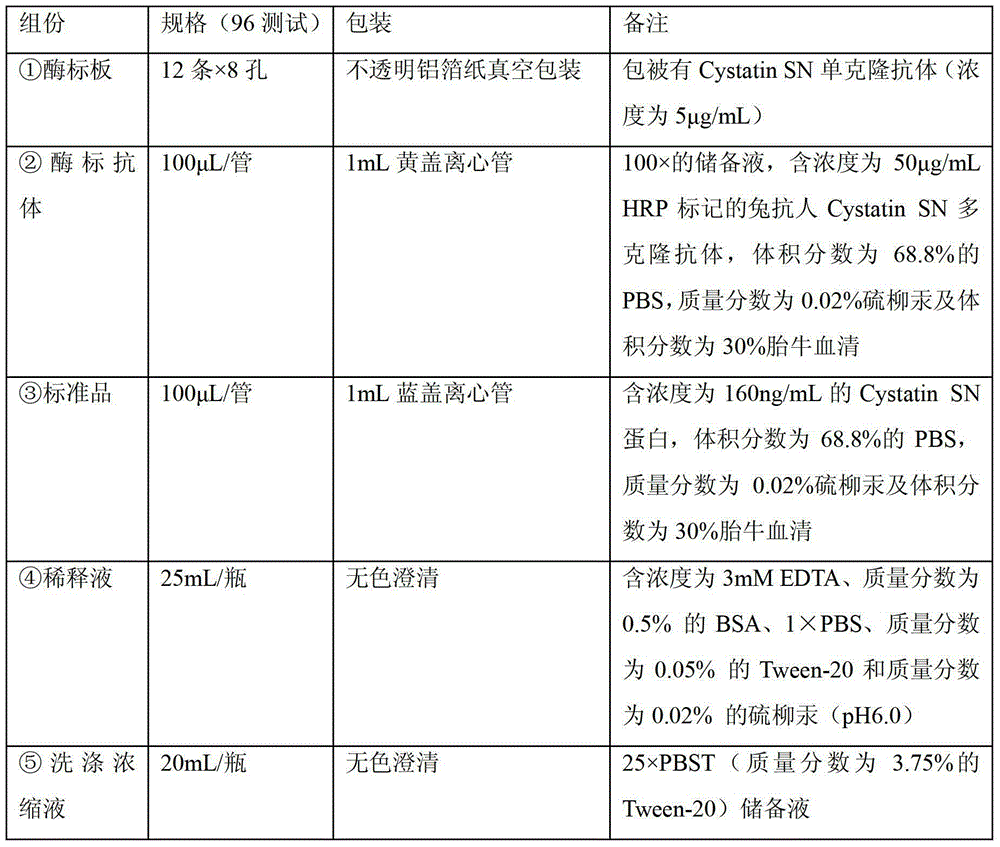
[0033] According to the Cystatin SN serum detection system established in Example 1, the Cystatin SN enzyme-linked immunoassay kit was constructed, and the specific components are as shown in Table 1:
[0034] Table 1. Cystatin SN ELISA kit components
[0035]
[0036]
[0037] Evaluation of Cystatin SN ELISA kit: use Cystatin SN ELISA kit to detect Cystatin SN positive quality control products, and repeat the detection 10 times at two levels of Cystatin SN protein concentration of 160pg / mL and 80pg / mL respectively, the results It shows that the coefficient of variation CV≤10%; if the same sample is tested with 3 batches of kits, the inter-assay coefficient of variation CV of the 3 batches of kits is ≤15%. The research on the stability of the kit shows that it can be stored at 4°C for 8 months, stored at 4°C for 2 months after opening, and transported at 0-4°C for 7 days.
Embodiment 3A
[0038] Example 3 AFP enzyme-linked immunosorbent assay (ELISA) detection kit
[0039] The AFP-ELISA detection kit uses the product of Beijing Rejing Biotechnology Co., Ltd. (Cat. No. 1003). The amino acid sequence of the detected AFP marker is shown in SEQ ID NO.2.
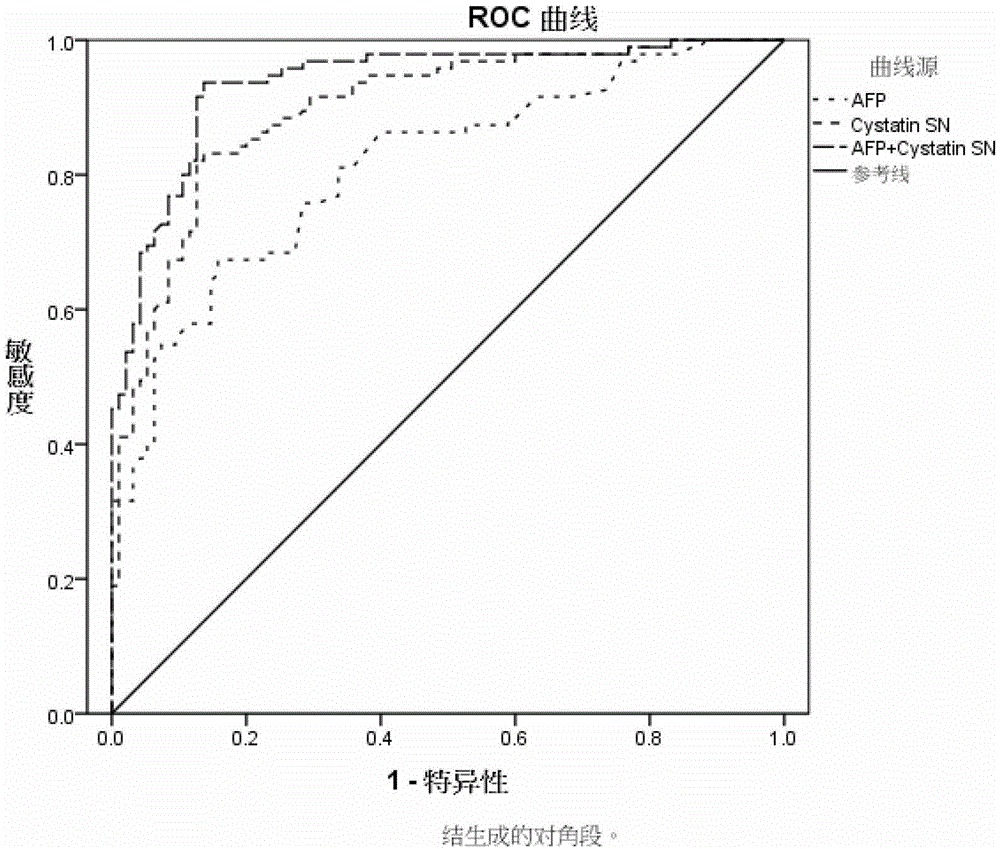
[0040] The Cystatin SN enzyme-linked immunoassay kit constructed in Example 2 was combined with the AFP-ELISA detection kit in this example to form a Cystatin SN-AFP combined detection kit.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com