Fusion protein efficiently combined with PD-1 and VEGF, and coding sequence and use thereof
A technology of fusion protein and PD-L2, applied in the field of fusion protein, can solve problems such as unaffordable for patients, redundant signaling pathways, and insufficient curative effect of single target therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: Design and synthesis of fusion protein expression cassettes and construction of expression vectors
[0088] According to the amino acid sequence and the coding sequence of each component of the fusion protein, the amino acid sequence and the coding DNA expression frame of the whole fusion are spliced into, wherein:
[0089] The amino acid residue sequence of the extracellular domain of PD-L2 is:
[0090] LFTVTVPKELYIIEHGSNVTLECNFDTGSHVNLGAITASLQKVENDTSPHRERATLLEEQLPLGKASFHIPQVQVRDEGQYQCIIIYGVAWDYKYLTLKVKASYRKINTHILKVPETDEVELTCQATGYPLAEVSWPNVSVPANTSHRSRTPEGLYQVTSVLRLKPPPGRNOWSCQFWNTHQVRELTLAS
[0091] The coding sequence of the extracellular region of PD-L2 is:
[0092] CTCTTTACTGTGACCGTGCCAAAAGAACTGTATATCATTGAGCACGGGTCCAATGTGACCCTCGAATGTAACTTTGACACCGGCAGCCACGTTAACCTGGGGGCCATCACTGCCAGCTTGCAAAAAGTTGAAAACGACACTTCACCTCACCGGGAGAGGGCAACCCTCTTGGAGGAGCAACTGCCATTGGGGAAGGCCTCCTTTCATATCCCTCAGGTGCAGGTTCGGGATGAGGGACAGTACCAGTGCATTATTATCTACGGCGTGGCTTGGGATTACAAGTATCTGAC...
Embodiment 2
[0134] Embodiment 2: Expression and purification of fusion protein
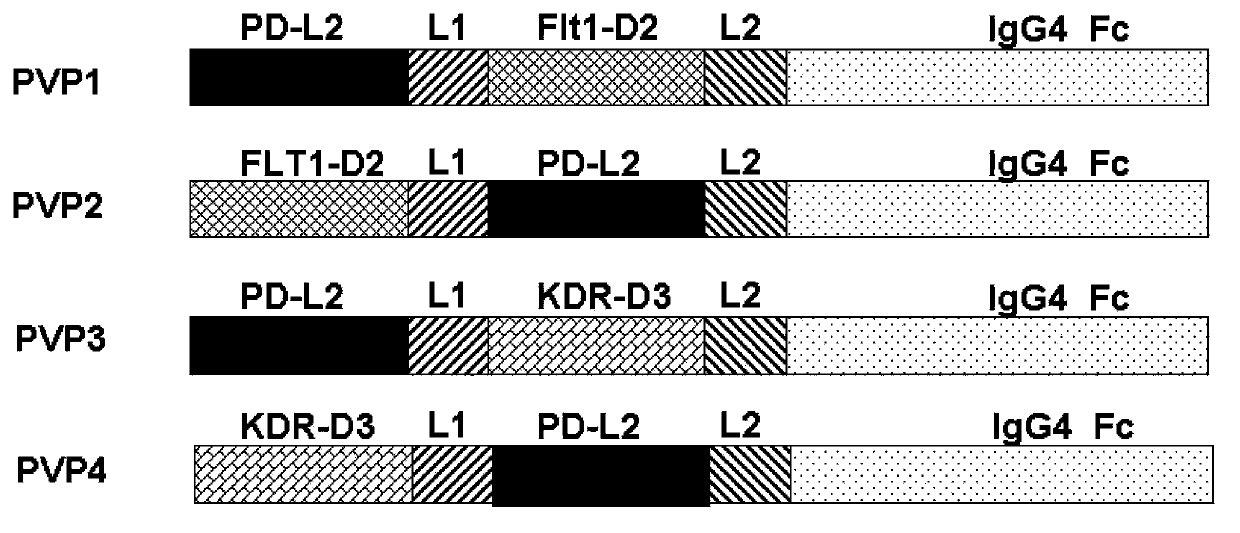
[0135] The high-quality plasmids of each recombinant expression vector constructed and purified in Example 1 were transfected into 293 cells (embryonic kidney cells, purchased from American Type Collection, ATCC) using Lipofectamine2000 (Invitrogen). After 2 days, the transfected 293 cells were transferred to DMEM medium with neomycin, and the cells were cloned by limiting dilution method. After 21 days of selection, a neomycin-resistant cell line stably transfected with the corresponding expression vector was established. Then, a large number of stably transfected cells were expanded by shaking flask culture, the culture supernatant was collected, and each fusion protein was purified by gel filtration affinity chromatography. The molecular weights of PVP1, PVP2, PVP3, and PVP4 are 73.3KD, 73.3KD, 72.7KD, and 72.7KD, respectively, and the concentration of the purified fusion protein was determined by ELISA...
Embodiment 3
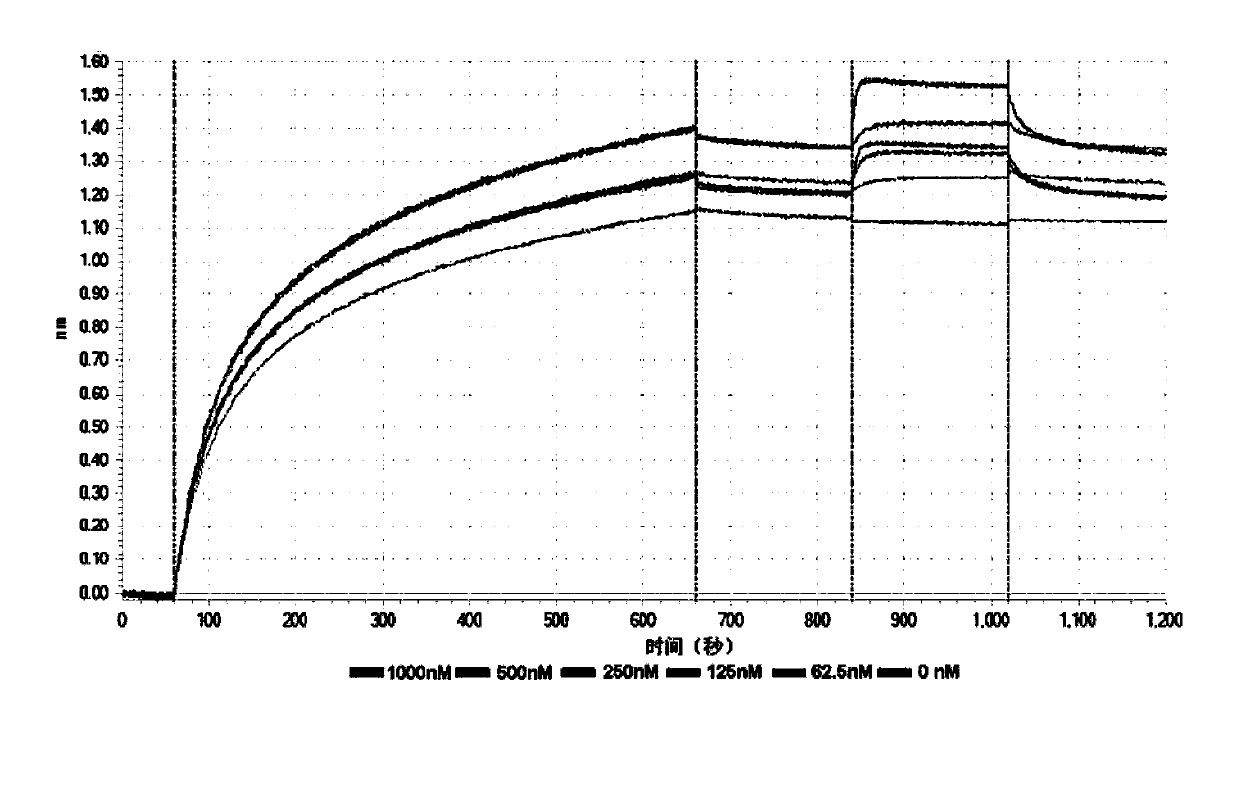
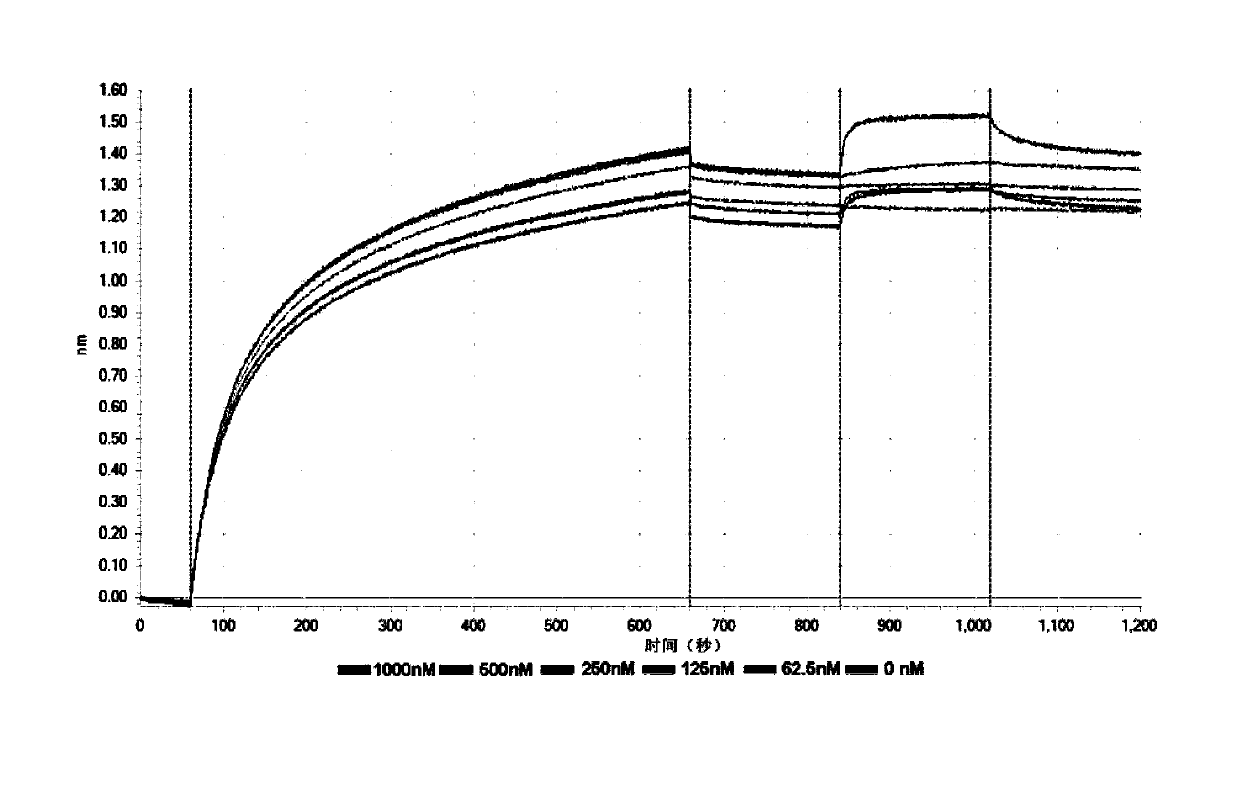
[0136] Example 3: Determination of the affinity constant of the fusion protein with PD-1 and VEGF
[0137] The fusion protein PVP1 prepared in Example 2 was added to a 96-well plate (10 μg / ml, 200 μl), and then loaded onto an anti-human IgG Fc capture biosensor (Anti-Human IgG Fc Capture biosensor, Octet), and then applied to a biomacromolecule The interaction instrument (Octet Red96) was used to measure its affinity with different concentrations (1000nM, 500nM, 250nM, 125nM, 62.5nM, 0nM) of recombinant human PD-1 and VEGF protein (200μl, SinoBiological lnc.), and the measured PVP1 and The affinity constants Kd of PD-1 and VEGF are 97.3nM respectively (see figure 2 ) and 30.2nM (see image 3 ). Those skilled in the art know that generally Kd is less than 10 -7 (That is, 100nM, the smaller the affinity, the higher the affinity), it means that the two proteins can be combined efficiently. Of course, this affinity is still relatively low compared to the affinity between an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com