Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
A phosphorescent material, metal iridium technology, applied in luminescent materials, electro-solid devices, organic chemistry, etc., can solve the problems of low luminous efficiency, high luminescent color purity, poor stability of light-emitting devices, and reduce self-quenching phenomenon. , the effect of high luminous efficiency and broad commercial development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
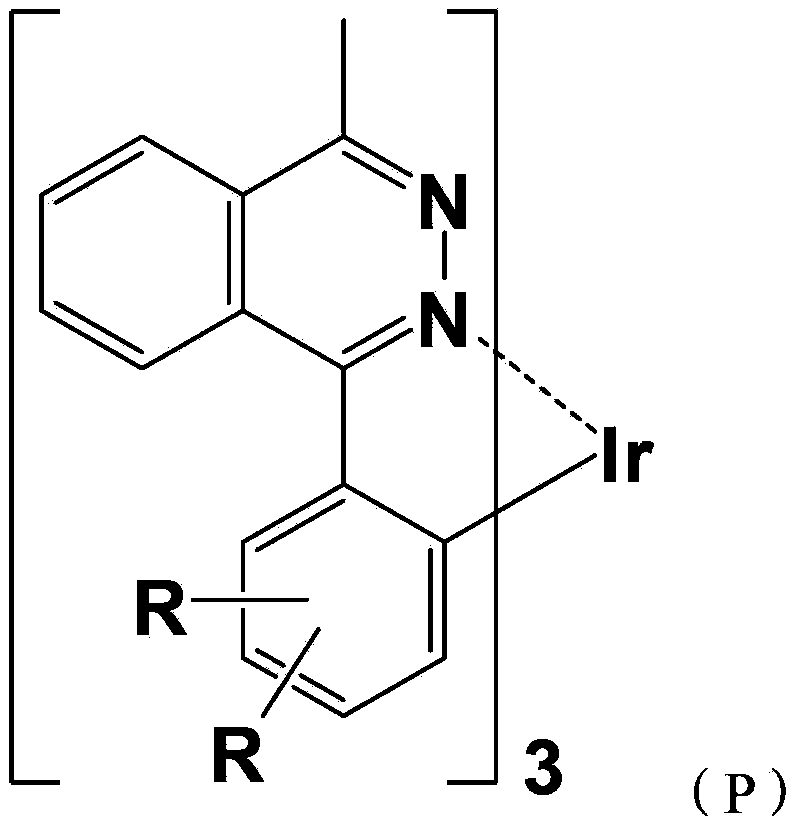
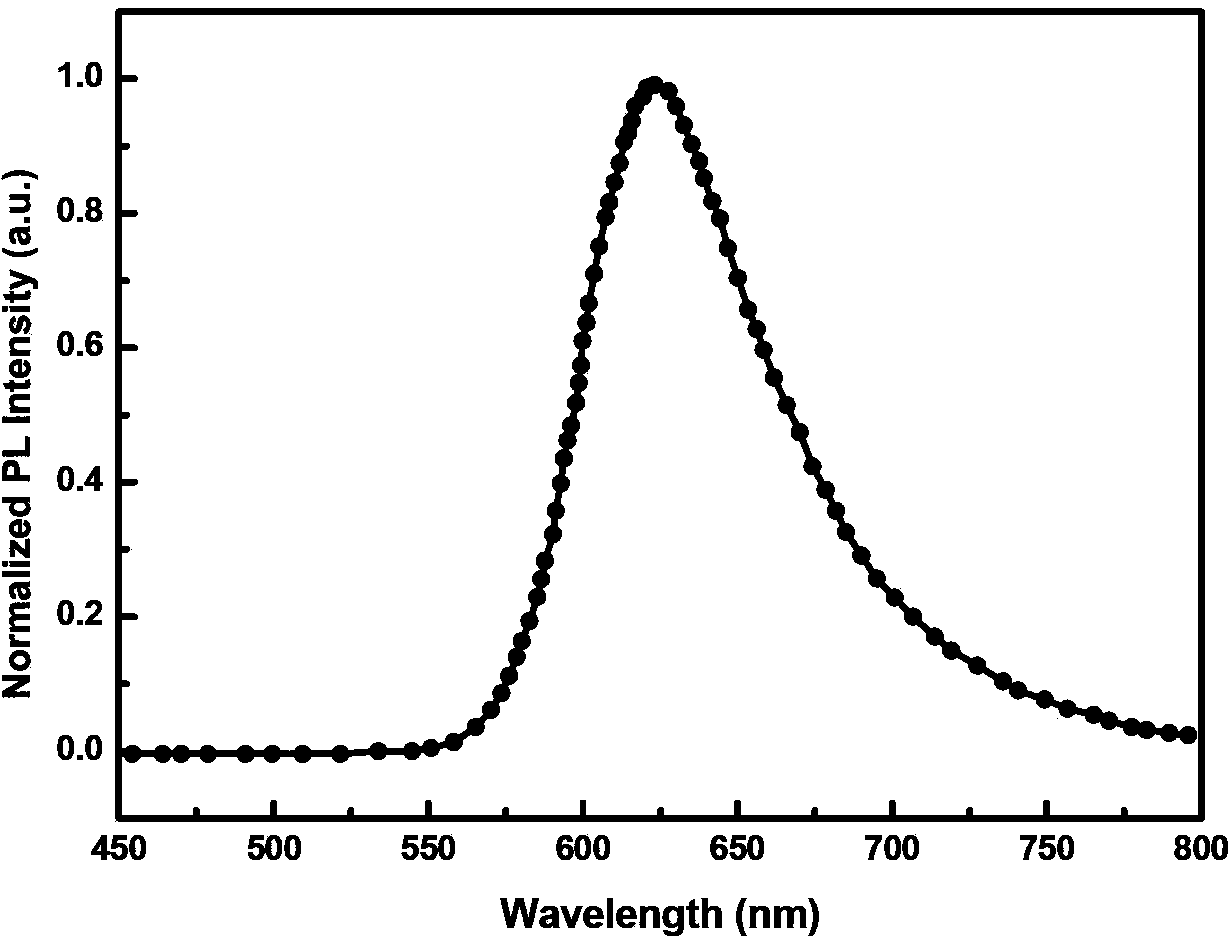
[0058] A red-light organic electrophosphorescent material metal iridium complex tris[1-methyl-4-(4',6'-dimethylphenyl)phthalazine-N,C 2 ']iridium, with 1-methyl-4-(2',4'-dimethylphenyl)phthalazine as the ring metal ligand, the structural formula is as follows:
[0059]
[0060] Wherein, the ring metal ligand is:
[0061]
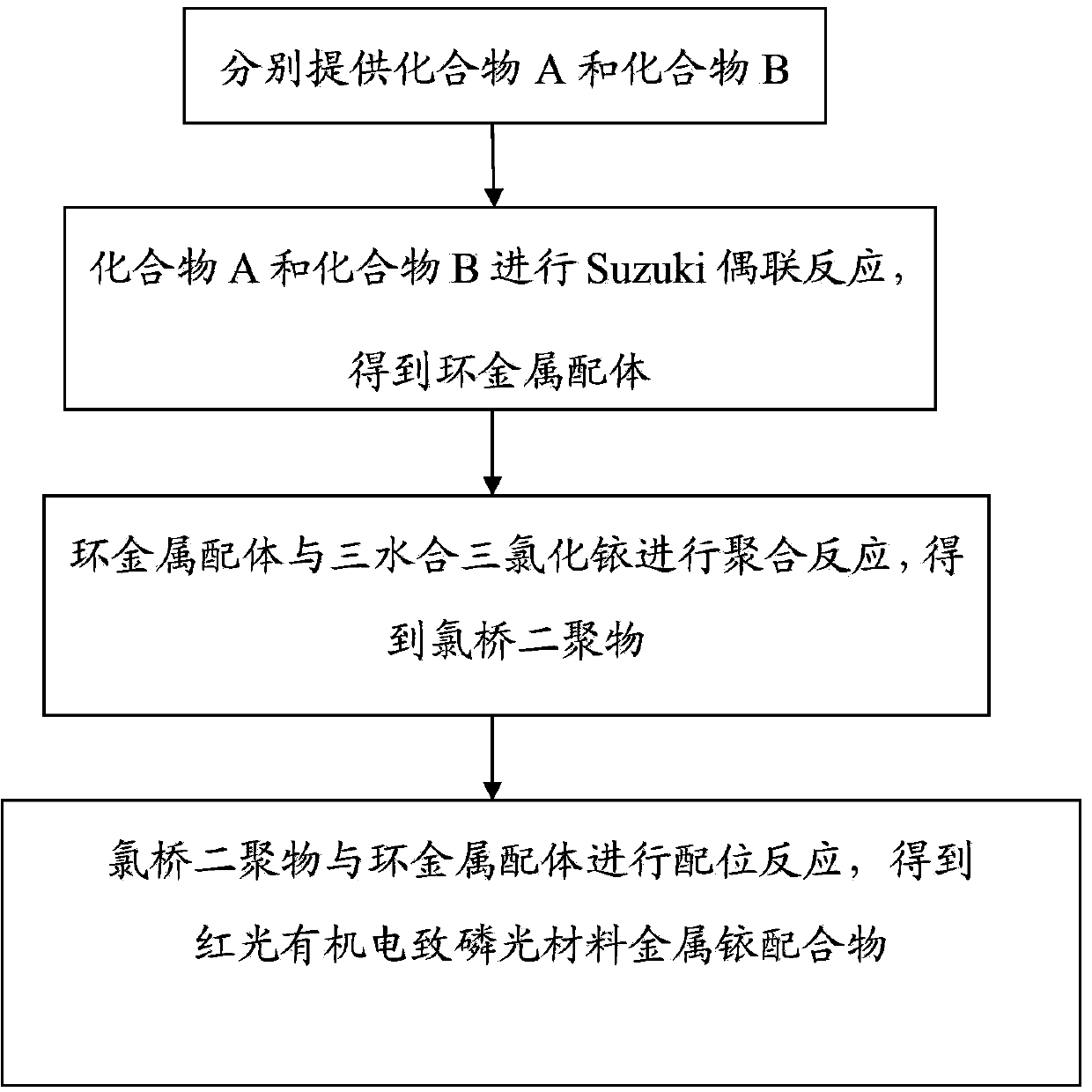
[0062] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0063] (1) Provide compound A and compound B1 represented by the following structural formula respectively:
[0064]
[0065] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',4'-dimethylphenyl)phthalazine
[0066]
[0067] Under nitrogen protection, 15mL of dimethylformamide (DMF) solution was added to the reactor, and then 0.72g (4.0mmol) of compound A (1-methyl-4-chlorophthalazine), 0.72g (4.8mmol ) Compound B1 (2,4-dimethylphenylboronic acid) and 0.23g (0.20mmol) tetrakis(triphe...
Embodiment 2
[0089] A red-light organic electrophosphorescent material metal iridium complex tris[1-methyl-4-(5',6'-dimethylphenyl)phthalazine-N,C 2 ']iridium, with 1-methyl-4-(2',3'-dimethylphenyl)phthalazine as the ring metal ligand, the structural formula is as follows:
[0090]
[0091] Wherein, the ring metal ligand is:
[0092]
[0093] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0094] (1) Provide compound A and compound B2 represented by the following structural formula respectively:
[0095]
[0096] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',3'-dimethylphenyl)phthalazine
[0097]
[0098] Under nitrogen protection, 15mL of dimethylformamide (DMF) solution was added to the reactor, and then 0.72g (4.0mmol) of compound A (1-methyl-4-chlorophthalazine), 0.6g (4.0mmol ) Compound B2 (2,3-dimethylphenylboronic acid) and 0.28g (0.24mmol) tetrakis(triphen...
Embodiment 3
[0119] A red-light organic electrophosphorescent material metal iridium complex tris[1-methyl-4-(3',6'-dimethylphenyl)phthalazine-N,C 2 ']iridium, with 1-methyl-4-(2',5'-dimethylphenyl)phthalazine as the ring metal ligand, the structural formula is as follows:
[0120]
[0121] Wherein, the ring metal ligand is
[0122]
[0123] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0124] (1) Provide compound A and compound B3 represented by the following structural formula respectively:
[0125]
[0126] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',5'-dimethylphenyl)phthalazine
[0127]
[0128] Under nitrogen protection, 15mL of toluene was added to the reactor, and then 0.72g (4.0mmol) of compound A (1-methyl-4-chlorophthalazine), 1.20g (8mmol) of compound B3 (2,5-di Toluene boronic acid) and 0.28g (0.24mmol) tetrakis (triphenylphosphine) palladium are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com