Application of PCA3, CST1 and CST4 to preparation of prostate gland cancer marker and kit of PCA3, CST1 and CST4
A prostate cancer and reagent kit technology, applied in the field of diagnosis, can solve the problems of prostate cancer diagnosis and prediction, curative effect evaluation, metastasis and recurrence monitoring, etc., and achieve the effect of reducing sampling pain, good specificity, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
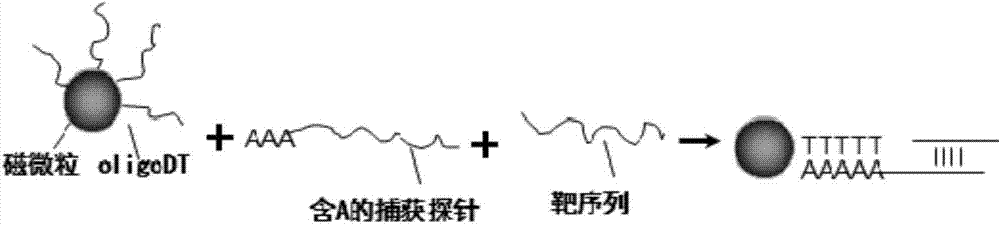
[0029] Embodiment 1, magnetic bead method specifically enriches the mRNA of PCA3, CST1, CST4 and PSA gene from urine
[0030]According to the mRNA sequence design of PCA3, CST1, CST4 and PSA, the specific probe (the nucleotide sequence of PCA3mRNA is shown in SEQ ID NO.1, the nucleotide sequence of CST1mRNA is shown in the mRNA of PCA3, CST1, CST4 and SDHA gene) Shown in SEQ ID NO.2, the nucleotide sequence of CST4mRNA is as SEQ ID NO.3, and the nucleotide sequence of PSA mRNA is as SEQ ID NO.4), specifically as follows: the probe for capturing PCA3mRNA is 5'-atctgttttcctgcccatcctttaagttta( dA)30-3'(SEQ ID NO.5), the probe for capturing CST1mRNA is 5'-aaagagcacaactgtttcttctgca(dA)30-3'(SEQ ID NO.6), the probe for capturing CST4mRNA is 5'-taccaggtctattagaagca( dA) 30-3' (SEQ ID NO.7), the probe for capturing the internal reference gene PSA mRNA is 5' cgaacttgcgcacacacgtcattggattta (dA) 30-3' (SEQ ID NO.8), the above-mentioned specific probe can be combined with magnetic beads (...
Embodiment 2
[0055] Example 2, Detection of the expression of CST1 and CST4 in prostate cancer tissue
[0056] 30 prostate cancer and adjacent tissue samples were collected from the Urology Department of Shanghai Fifth People's Hospital, cut to the size of rice grains, stored in RNAlater preservation solution at -80°C, and equilibrated to room temperature before use. Then according to the method of Example 1, the CST1mRNA, CST4mRNA and PSA mRNA of the paired tissue of prostate cancer and adjacent cancer were respectively enriched, and then the detection primers of CST1, CST4 and internal reference gene PSA detection primer were used to detect 30 samples of prostate cancer and adjacent cancer. The relative expression levels of CST1 gene and CST4 gene in paired tissues, the detection system and detection conditions are the same as those in Example 1. The test results are 2 -ΔΔCP The relative expression was calculated by the method, and then the ratio (C / N) of the relative expression between...
Embodiment 3
[0057] Embodiment 3, construction prostate cancer detection kit
[0058] 1. Construction of PCA3 recombinant plasmid
[0059] The prostate cancer cell line LNcap was used as the material to extract the total mRNA of the prostate cancer cell line LNcap, and then the extracted mRNA was used as a template to synthesize cDNA, and the primers for constructing the PCA3 recombinant plasmid were designed according to the PCA3 gene sequence, and the upstream primer was 5'-gccgagggagaccaggaagat-3' (SEQ ID NO.17), the downstream primer is 5'-ggcccagaaggaaccgtagag-3' (SEQ ID NO.17), with the nucleotides shown in SEQ ID NO.17 and SEQ ID NO.18 as primers, the synthetic cDNA is The template was amplified by PCR, and the amplification conditions were: denaturation at 94°C for 5 minutes; 45 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 1 minute; extension at 72°C for 5 minutes, and cooling at 4°C. The amplified product was connected...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com