Pharmaceutical composition for treating bronchial asthma and application thereof
A technology for bronchial asthma and a composition, which is applied in the field of medicine to achieve the effects of improving lung function, reducing dosage and reducing airway responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following are specific examples of the present invention, and further describe the technical solution of the present invention, but the protection scope of the present invention is not limited to these examples. All changes or equivalent substitutions that do not depart from the concept of the present invention are included in the protection scope of the present invention.
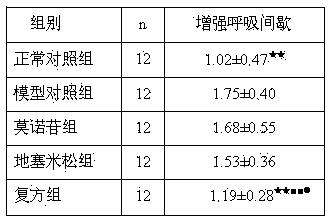
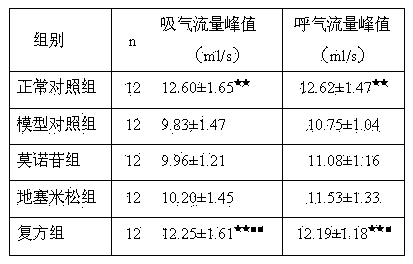
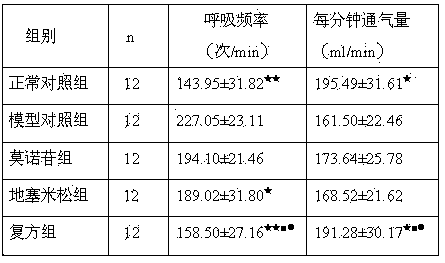
[0018] 60 clean SD rats, half male and half male, body weight 180-220g. All rats were randomly divided into normal control group, model control group, dexamethasone group, morroniside group and compound group, 12 in each group. Animal models of bronchial asthma were prepared by ovalbumin (OVA) sensitization. Except for the normal control group, animals in the other four groups were treated with OVA 10 mg, A1(OH) 3 Dry powder 100mg normal saline suspension 1mL intraperitoneal injection for sensitization, repeat sensitization once on the 8th day, start ultrasonic atomization 1% 0VA for excitatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com