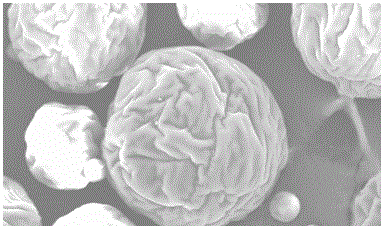
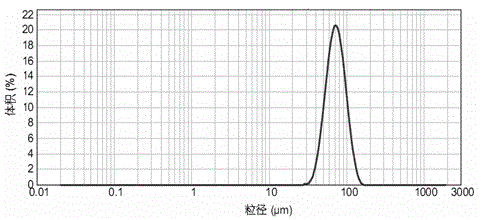
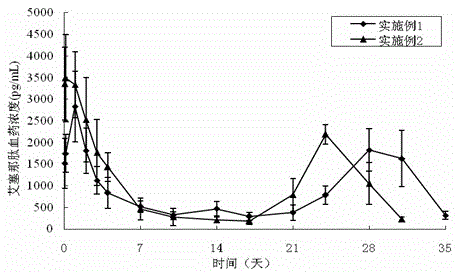
A kind of exenatide sustained-release microsphere composition
A technology of exenatide and slow-release microspheres, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of single PLG5% drug-loaded exenatide microspheres
[0049] Weigh 0.2g Exenatide Acetate and put it into a 5ml EP tube according to the dried and pure 0.2g Exenatide, weigh another 0.08g sucrose, put it into the EP Tube, add 2.4g Water for Injection, vortex to dissolve and put it in ice bath Save for later. Weigh 3.72g of PLG50504A (Evonik Industries AG, intrinsic viscosity 0.41dL / g, LA:GA50:50, weight average molecular weight about 55000Da), put it into a 50ml glass vial, and weigh 58.3g of dichloromethane, add it to the glass vial to dissolve, and put it in an ice bath Save for later. Using an ultrasonic cell breaker with a power of 1600W, under 40% amplitude ultrasonic, add the aqueous phase into the dichloromethane solution with a syringe, ultrasonic for 1 min, then stop for 1 min, and then ultrasonic for 2 min to form colostrum.
[0050] Place a 250ml beaker in a 3°C constant temperature water bath, transfer the colostrum into the beaker, an...
Embodiment 2
[0052] Example 2 Preparation of single PLG10% drug-loaded exenatide microspheres
[0053] Weigh 0.4g of exenatide after dehydration and purification, put it into a 5ml EP tube, weigh 0.16g of sucrose, put it into an EP tube, add 2.4g of water for injection, vortex to dissolve, and then ice-bath Save for later. Weigh 3.44g of PLG50504A (Evonik Industries AG, intrinsic viscosity 0.41dL / g, LA:GA50:50, weight average molecular weight about 55000Da), put it into a 50ml glass vial, and weigh 58.3g of dichloromethane, add it to the glass vial to dissolve, and put it in an ice bath Save for later. Using an ultrasonic cell breaker with a power of 1600W, under 40% amplitude ultrasonic, add the aqueous phase into the dichloromethane solution with a syringe, ultrasonic for 1 min, then stop for 1 min, and then ultrasonic for 2 min to form colostrum.
[0054] Place a 250ml beaker in a 3°C constant temperature water bath, transfer the colostrum into the beaker, and stir with a mechanical s...
Embodiment 3
[0056] Example 3 Preparation of two kinds of PLG10% drug-loaded exenatide microspheres
[0057] Weigh 0.42g of exenatide after dry and pure, put it into a 5ml EP tube, weigh another 0.16g of sucrose, put it into an EP tube, add 2.4g of water for injection, vortex to dissolve, and put it in ice bath Save for later. Weigh 0.855g PLG50501A (Evonik Industries AG, intrinsic viscosity 0.11dL / g, LA:GA50:50, weight average molecular weight about 5500Da), 2.565g PLG50504A (Evonik Industries AG, intrinsic viscosity 0.41dL / g, LA:GA50:50, weight average molecular weight about 55000Da ), put it into a 50ml glass vial, weigh another 58.3g of dichloromethane, add it into the glass vial to dissolve, and store it in an ice bath for later use. Using an ultrasonic cell breaker with a power of 1600W, under 40% amplitude ultrasonic, add the aqueous phase into the dichloromethane solution with a syringe, ultrasonic for 1 min, then stop for 1 min, and then ultrasonic for 2 min to form colostrum.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com