DNA molecule, recombinant plasmid and recombinant bacterium for production of D-p-Hydroxyphenylglycine
A technology of p-hydroxyphenylglycine and DNA molecules, applied in the field of DNA molecules, can solve problems such as handling problems, and achieve the effects of environmental friendliness, simple operation and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, construction of recombinant plasmid
[0044] 1. Construction of recombinant plasmid pUC-hyd-car
[0045] 1. Synthesize the double-stranded DNA molecule shown in sequence 2 of the sequence listing.
[0046] 2. Using the double-stranded DNA molecule synthesized in step 1 as a template, perform PCR amplification with a primer pair consisting of hyd-for and hyd-rev to obtain a PCR amplification product.
[0047] hyd-for: 5'-TGCTT GTC GAC ATGAGCACTGTCATCAAGGG-3';
[0048] hyd-rev: 5'-ACGAAC TCTAGA TCAGACGCCGCTTGCGGGAAT-3'.
[0049] 3. The PCR amplified product of step 2 was double-digested with restriction enzymes Sal I and Xba I, and the digested product was recovered.
[0050] 4. Digest the vector pUC18 with restriction endonucleases Sal I and Xba I to recover the vector backbone (about 2680 bp).
[0051] 5. Ligate the digested product of step 3 with the vector backbone of step 4 to obtain the recombinant plasmid pUC-hyd.
[0052] 6. Synthesize the d...
Embodiment 2
[0087] Embodiment 2, the preparation of recombinant bacteria
[0088] The recombinant plasmid pUC-pPL-hyd-car was introduced into Escherichia coli DH5α competent cells to obtain recombinant bacteria DH5α / pUC-pPL-hyd-car.
[0089] The recombinant plasmid pUC-pPAND-hyd-car was introduced into Escherichia coli DH5α competent cells to obtain recombinant bacteria DH5α / pUC-pPAND-hyd-car.
[0090] The recombinant plasmid pUC-pT7A1-hyd-car was introduced into Escherichia coli DH5α competent cells to obtain recombinant bacteria DH5α / pUC-pT7A1-hyd-car.
Embodiment 3
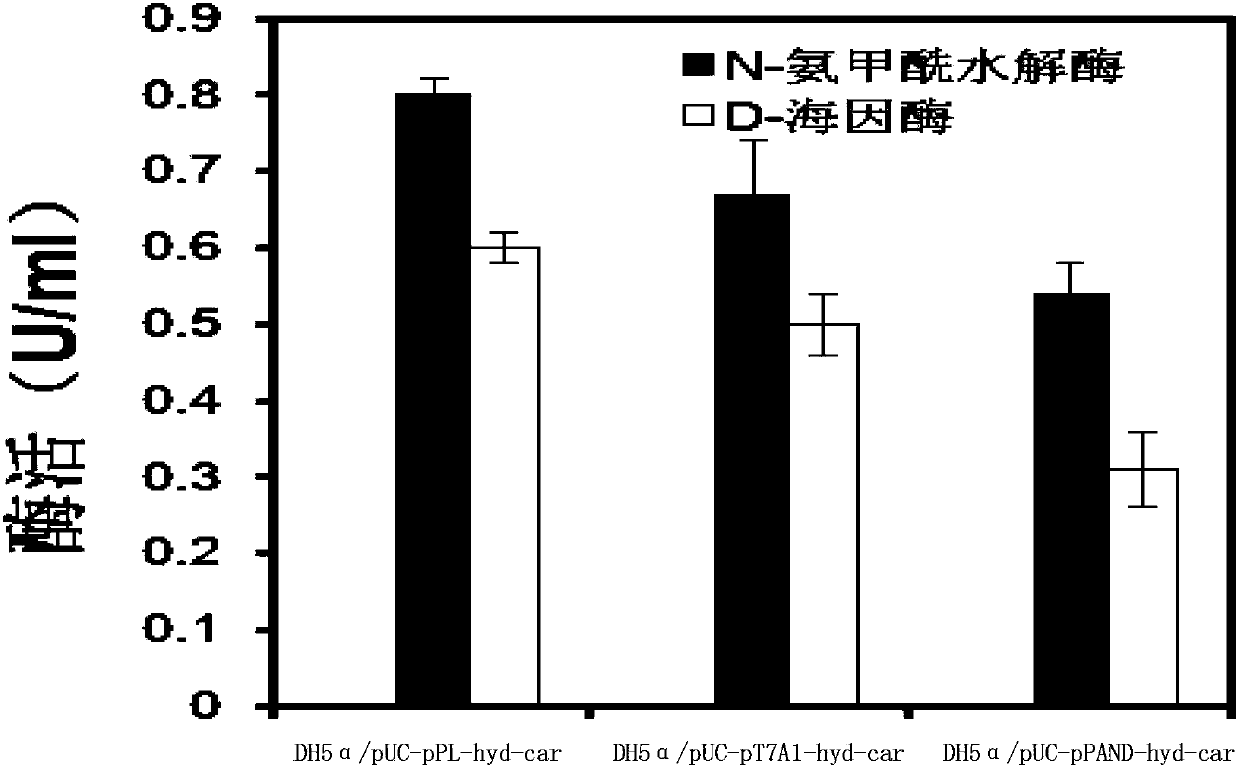
[0091] Embodiment 3, the comparison of different recombinant bacteria expression target protein levels
[0092] Each recombinant bacterium prepared in Example 2 was carried out as follows:
[0093] 1. Pick a single colony of the recombinant bacteria and inoculate it in 15ml of LB liquid medium containing 100μg / ml ampicillin, culture at 37°C with shaking at 200rpm, and obtain OD 600nm =2.8 bacteria solution.
[0094]2. Take 10ml of the bacterial liquid obtained in step 1 and transfer it to 100ml of LB liquid medium containing 100μg / ml ampicillin to obtain OD 600nm =3.0 initial fermentation system, 37 ° C, 200 rpm, shaking culture for 12 hours, to obtain a termination fermentation system.
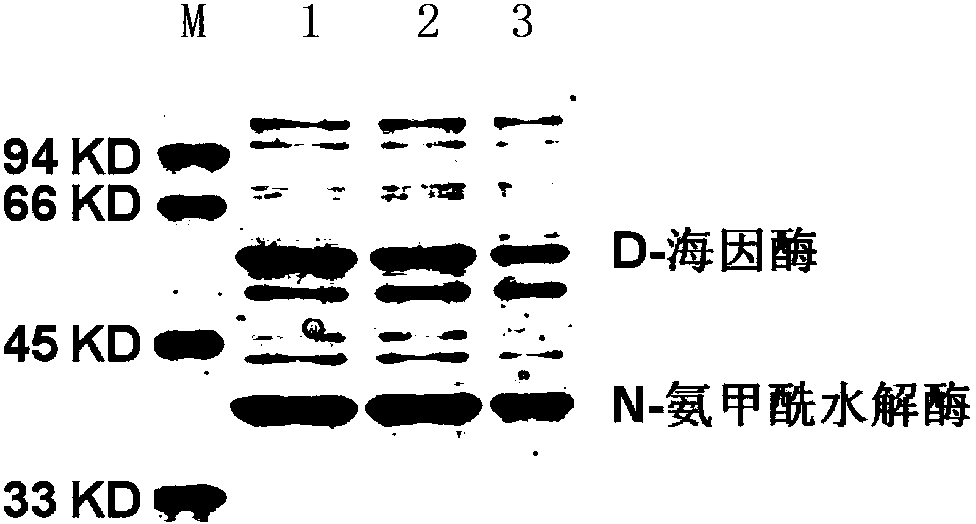
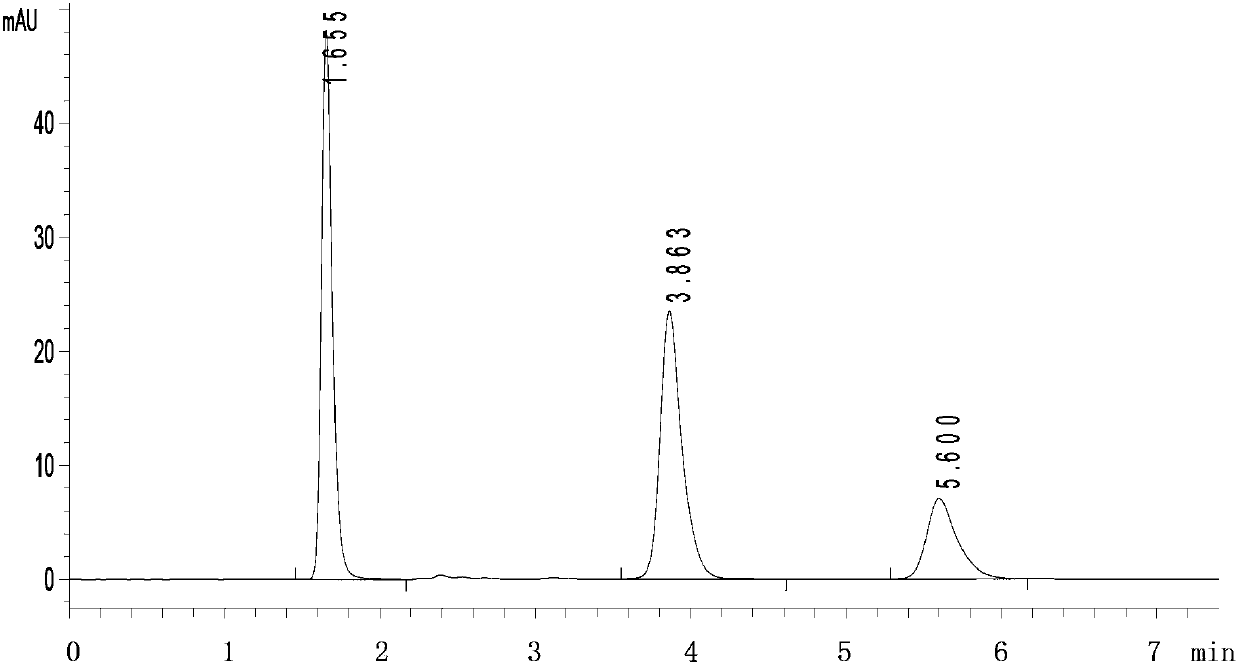
[0095] 3. Take 2mL to terminate the fermentation system, centrifuge at 10,000rpm for 1min, collect the bacterial precipitate, ultrasonically break (ultrasonic power is 200w, processing time is 4min, stop for 3s every 3s), then centrifuge at 10,000rpm for 1min, and collect the supernatant. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com