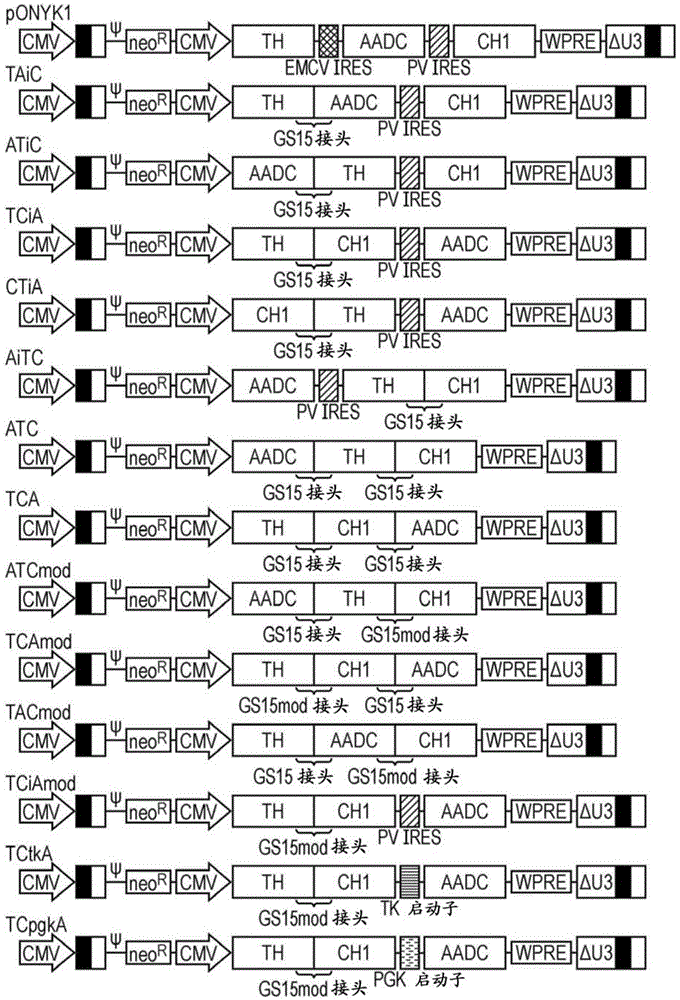
Construct
A technology of constructs and viral vectors, applied in the field of constructs, can solve the problem that the titer does not have an inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0260] Example 1 - Generation of vector from pONYK-TAiC and generation of catecholamines from integrated vector
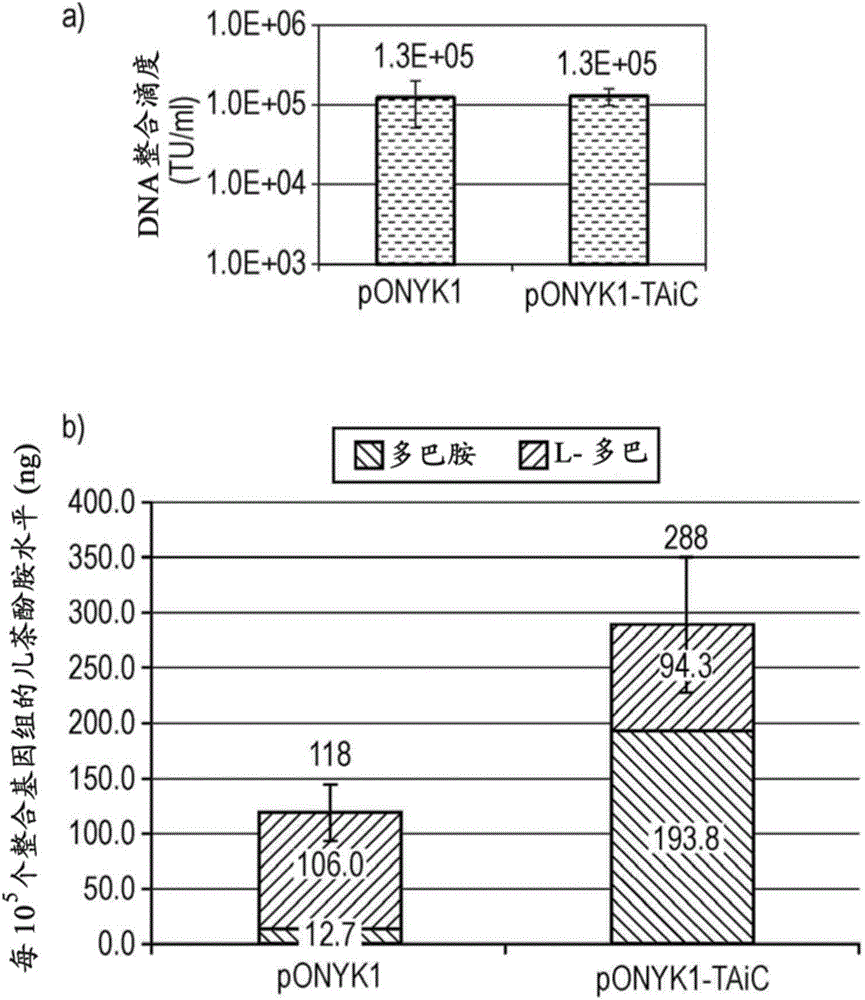
[0261] The first fusion construct to be generated and tested for improved titers compared to pONYK1 was pONYK-TAiC( figure 1 ). Lentiviral vector (LV) preparations were generated in triplicate using the pONYK-TAiC or pONYK1 genomes and the titers of the resulting vectors were quantified by DNA integration assays ( figure 2 a). These data surprisingly show that the titer of the vector generated from pONYK-TAiC is the same as pONYK1, ie removal of 1 IRES element does not improve the titer. HPLC analysis was performed on transduced HEK293T cell supernatants to examine the levels of L-DOPA and dopamine produced. HPLC results ( figure 2 b) shows that cells transformed with the pONYK-TAiC vector produced a 2.4-fold increase in total catecholamine levels compared to cells transduced with the pONYK1 vector. L-DOPA levels were comparable between the two genomes, h...
Embodiment 2
[0262] Example 2 - Vector Production and Production of Catecholamines from Integrated Vectors
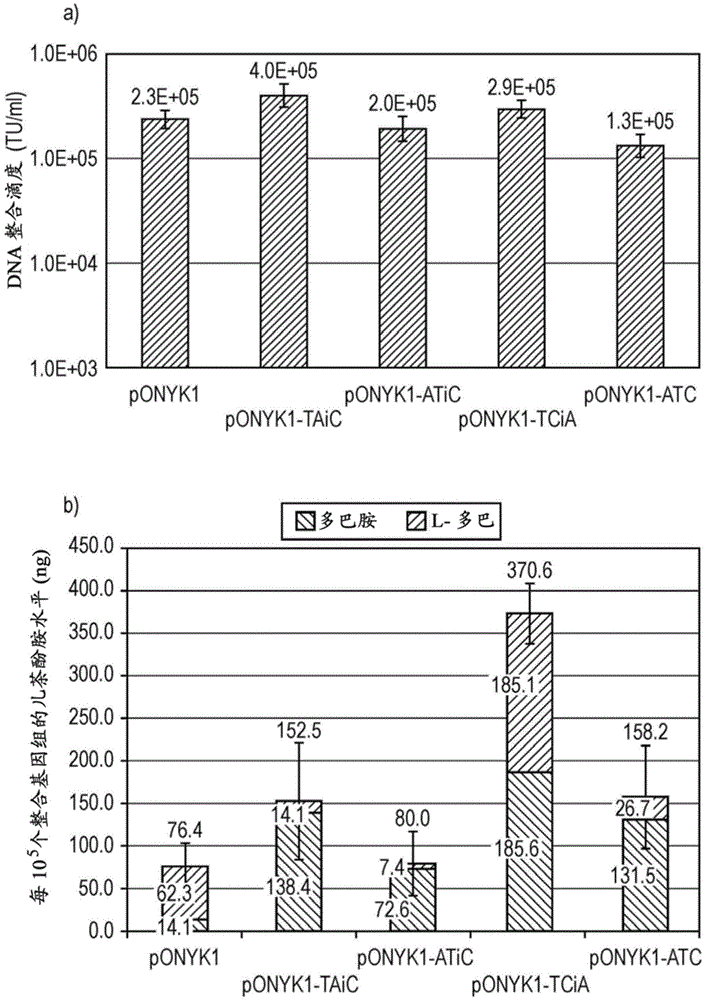
[0263] Together with pONYK-TAiC and pONYK1, three other dopaminease fusion plasmids (pONYK-ATiC, pONYK-TCiA, pONYK-ATC( figure 1 ). LV preparations were generated in triplicate using each of the different genomic plasmids and the titers of the resulting vectors were quantified by DNA integration analysis ( image 3 a). The results showed that the titers were similar for all vectors, ranging from 1.3E+05TU / ml to 4.0E+05TU / ml. Interestingly, pONYK-ATC lacking both IRES elements showed no increase in titer, suggesting that the fusion construct did not alter vector production and that the presence of the transgenic rearranged GS15 linker did not affect titer. The results of HPLC analysis showed that HEK293T cells transduced with each of the different vectors resulted in different catecholamine production ( image 3 b). Furthermore, the amount of L-DOPA converted to dopamine vari...
Embodiment 3
[0265] Example 3 - Evaluation of Dopaminerase Levels in HEK293T Cells Transfected with Different Fusion Plasmids
[0266] To investigate protein expression levels, western blot analysis of each transgene product (AADC, CH1, and TH) was performed from cell lysates of HEK293T cells that had been transfected with each fusion genome plasmid ( Figure 4 ). The results show that for each of the different fusion constructs, each dopamine synthetase is present and of the predicted size. This indicates that each genomic construct is capable of expressing dopamine synthase with a GS15 linker, including the triple fusion cassette (pONYK-ATC), as a 124 kDa band is visible in all three western blots, which is the dopamine enzyme containing all three links The expected size of the fusion protein of the protein. The levels of each of the different proteins expressed by the different genomic constructs varied widely. The highest levels of all three proteins appeared to be expressed from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com