Synthesis method of tetrabromobisphenol A analog-tetrabromobisphenol A hexanoic acid
A technology of tetrabromobisphenol and synthetic method, which is applied in the field of synthesis of tetrabromobisphenol A analog tetrabromobisphenol A hexanoic acid, can solve the problems of expensive instruments, long detection time, complicated sample processing, etc., and achieve the synthetic steps Concise, meet the needs of immune research, and the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
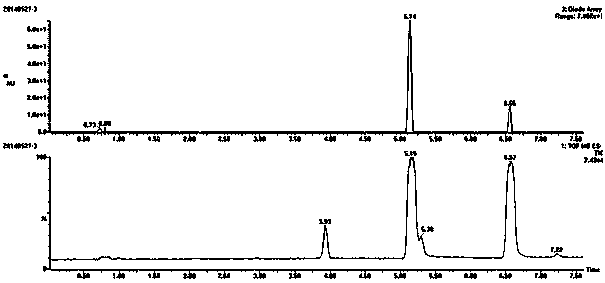
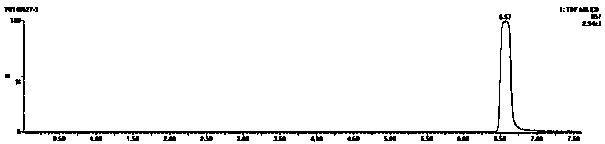
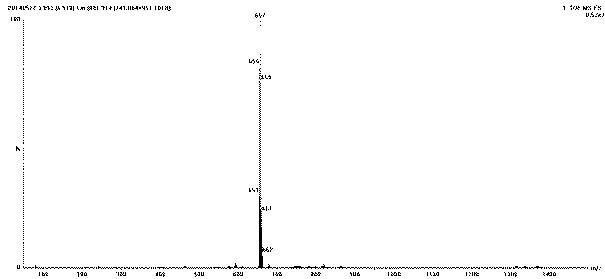
[0023] Add 1g, 1.84mmol of the compound tetrabromobisphenol A (TBBPA) into a 50mL round bottom flask, then add 20mL of N,N-dimethylformamide DMF, after the tetrabromobisphenol A is dissolved, add 3.68mmol 0.1M NaOH solution, placed at room temperature for later use. 1.84mmol of 6-bromohexanoic acid was dissolved in 3mL of DMF, then added dropwise to the mixed solution of tetrabromobisphenol A and NaOH, and reacted at 65°C for 2 hours. After the reaction, add water to the reaction solution, and adjust the pH to 1-2 with hydrochloric acid , obtained a precipitate, extracted with ethyl acetate, washed the organic phase three times with water, concentrated to obtain the product, and separated by column chromatography to obtain the target compound tetrabromobisphenol A hexanoic acid.
[0024] Characterization of tetrabromobisphenol A hexanoic acid:
[0025] The substance and purity identification of tetrabromobisphenol A hexanoic acid are characterized by means of ultra-high perfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com