Determination method of content of schisandrin in medicament
A technique for determination of schisandrin methyl alcohol is applied in the field of determination of the content of pharmaceutical components, which can solve the problems of long time, short detection time, difficult separation, etc., and achieve the effects of reducing usage, shortening detection time, and reducing analysis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A method for assaying content of Schisandrin A in medicine, comprising the following steps:
[0041] A. Preparation of test sample solution: Weigh 2.5g of the drug, grind it finely, place it in a Soxhlet extractor, add n-hexane to the extractor and soak it for 12 hours, reflux extraction at 80°C for 6 hours, and the extract is evaporated at low temperature. Dry, then add methanol slightly heat to dissolve, shake well, that is;
[0042] B. Preparation of reference substance solution: Weigh an appropriate amount of schisandra alcohol A reference substance, add methanol to make solutions containing 0.02mg, 0.05mg, 0.1mg, 0.2mg, and 0.5mg per 1ml, to obtain final product;
[0043] C. UPLC detection: the chromatographic column is C18, the mobile phase is methanol-water, the ratio of methanol-water is 72:28, and the flow rate is 0.30ml min -1 , the injection volume is 2μl, and the detection wavelength is 254nm;
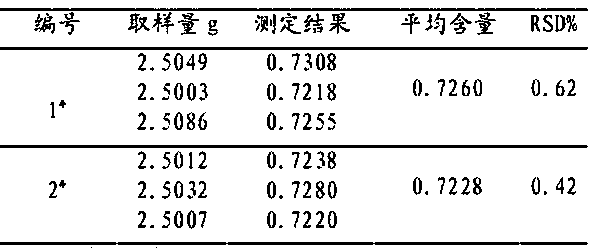
[0044] D, the formulation and calculation result of standard ...
Embodiment 2
[0046] A method for assaying content of Schisandrin A in medicine, comprising the following steps:
[0047] A. Preparation of test sample solution: Weigh 5g of the drug, grind it finely, add n-hexane to soak for 24 hours, reflux and extract at 85°C for 6 hours, evaporate the extract to dryness at low temperature, then add methanol to slightly heat to dissolve, shake well , that is;
[0048] B. Preparation of reference substance solution: Weigh an appropriate amount of schisandra alcohol A reference substance, add methanol to make a solution containing 0.02mg, 0.05mg, 0.1mg, 0.2mg, 0.5mg per 1ml, to obtain final product;
[0049] C. UPLC detection: the chromatographic column is C18, the mobile phase is methanol-water, the ratio of methanol-water is 72:28, and the flow rate is 0.30ml min -1 , the injection volume is 2μl, and the detection wavelength is 254nm;
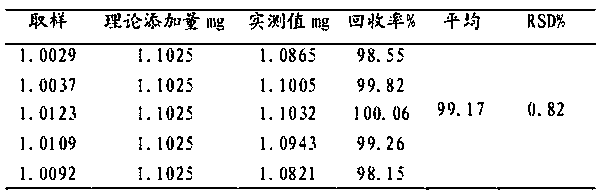
[0050] D, the formulation and calculation result of standard curve: the reference substance solution of each concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com