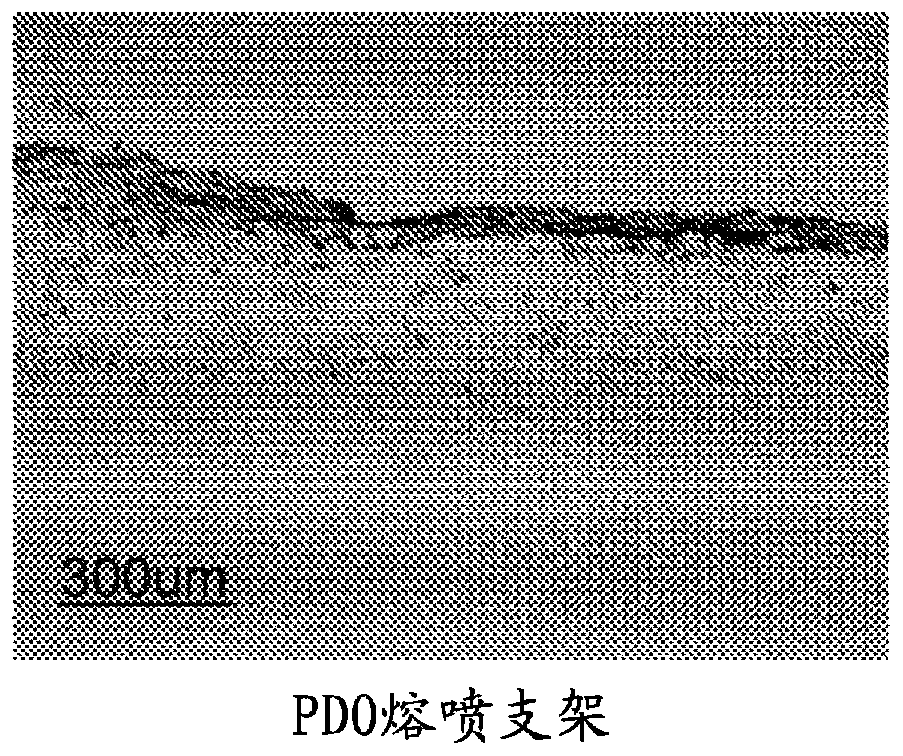
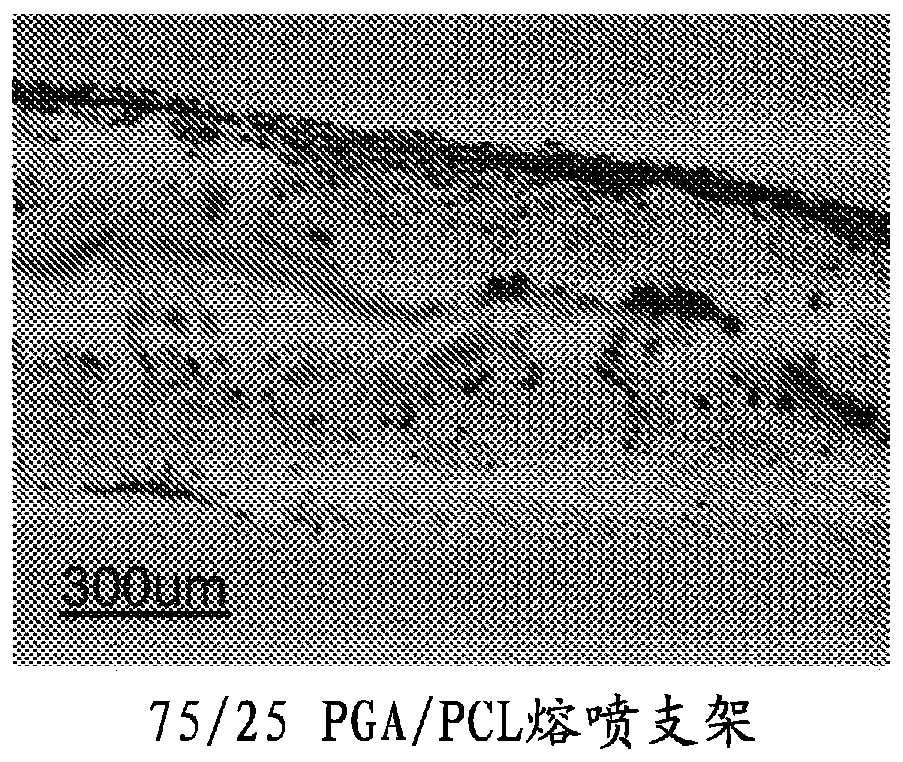
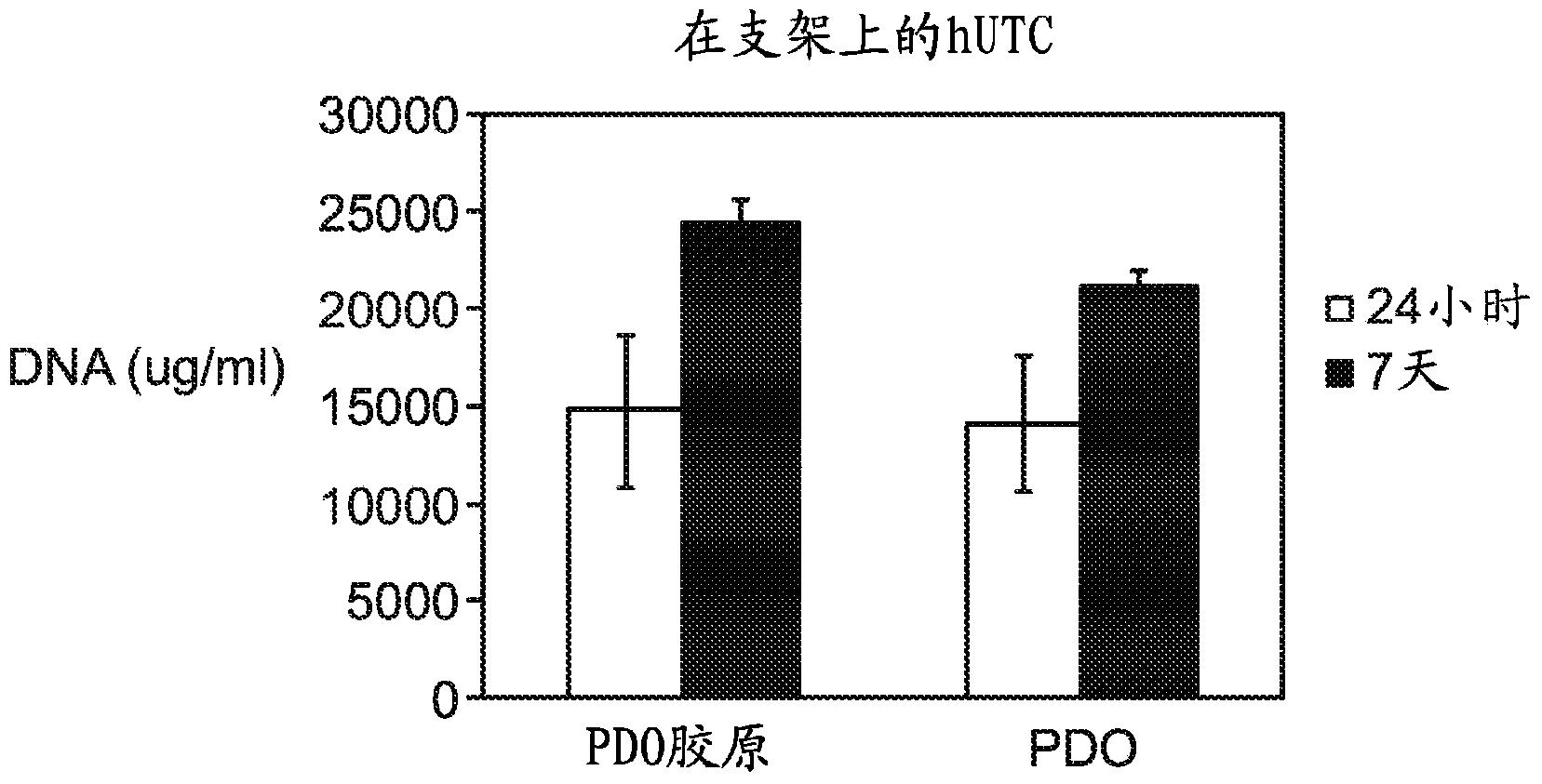
Segmented, epsilon-caprolactone-rich, poly(epsilon-caprolactone-co-p-dioxanone) copolymers for medical applications and devices therefrom
A technology of dioxanone and copolymer, which is applied in medical science, prosthesis, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0126] Example 1: Poly(ε-caprolactone-co- Synthesis of p-dioxanone) Triblock Copolymer
[0127] Using a 10 gallon stainless steel oil jacketed reactor equipped with agitation, 4,123 grams of ε-caprolactone was added along with 63.9 grams of diethylene glycol and 16.6 mL of a 0.33M stannous octoate solution in toluene. After the initial charge, a purge cycle was performed in an upward direction using agitation at a rotational speed of 6 RPM. The reactor was evacuated to a pressure of less than 550 mTorr, followed by introduction of nitrogen. The cycle is repeated one more time to ensure a dry atmosphere. At the end of the final nitrogen purge, the pressure was adjusted to slightly over one atmosphere. The vessel was heated at a rate of 180°C / hour by setting the oil controller at 195°C. The reaction lasted 6 hours and 10 minutes from the time the oil temperature reached 195°C.
[0128] In the next stage, the oil controller set point was lowered to 120°C and 20,877 grams ...
example 2
[0131] Example 2: Poly(ε-caprolactone-co-p Synthesis of Dioxanone) Triblock Copolymer (PDO-rich Cap / PDO Copolymer)
[0132] Using a 10 gallon stainless steel oil jacketed reactor equipped with agitation, 4,123 grams of ε-caprolactone was added along with 90.2 grams of diethylene glycol and 23.4 mL of a 0.33M stannous octoate solution in toluene. The reaction conditions in the first stage closely matched those in Example 1.
[0133] In the second copolymerization stage, the oil controller set point was lowered to 120°C and 32,089 grams of molten p-dioxanone monomer was added from the melt tank with stirring rotation at 7.5 RPM in a downward direction for 40 minutes . The oil controller was then set to 115°C for 20 minutes, then to 104°C for one hour and 45 minutes, and finally to 115°C for 15 minutes before draining. The post-curing phase (80°C / 4 days) and the grinding and sieving procedure were carried out according to Example 1. The ground and sieved polymer had a net ...
example 3
[0135] Example 3: Poly(ε-caprolactone-co-p-dioxa) enriched in ε-caprolactone in 91 / 9 molar segments Synthesis of cyclohexanone) triblock copolymer (Cap / PDO copolymer rich in Cap) [75 / 25 Initial feed charge for Cap / PDO]
[0136] Using a 10 gallon stainless steel oil jacketed reactor equipped with agitation, 18,492 grams of ε-caprolactone was added along with 19.1 grams of diethylene glycol and 26.2 mL of a 0.33M stannous octoate solution in toluene. After the initial charge, start the purge cycle in the down direction using agitation at a rotational speed of 10 RPM. The reactor was evacuated to a pressure of less than 500 mTorr, followed by introduction of nitrogen. The cycle is repeated one more time to ensure a dry atmosphere. At the end of the final nitrogen purge, the pressure was adjusted to slightly over one atmosphere. The rotational speed of the stirrer was reduced to 7 RPM in the downward direction. The vessel was heated at a rate of 180°C / hour by setting the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com