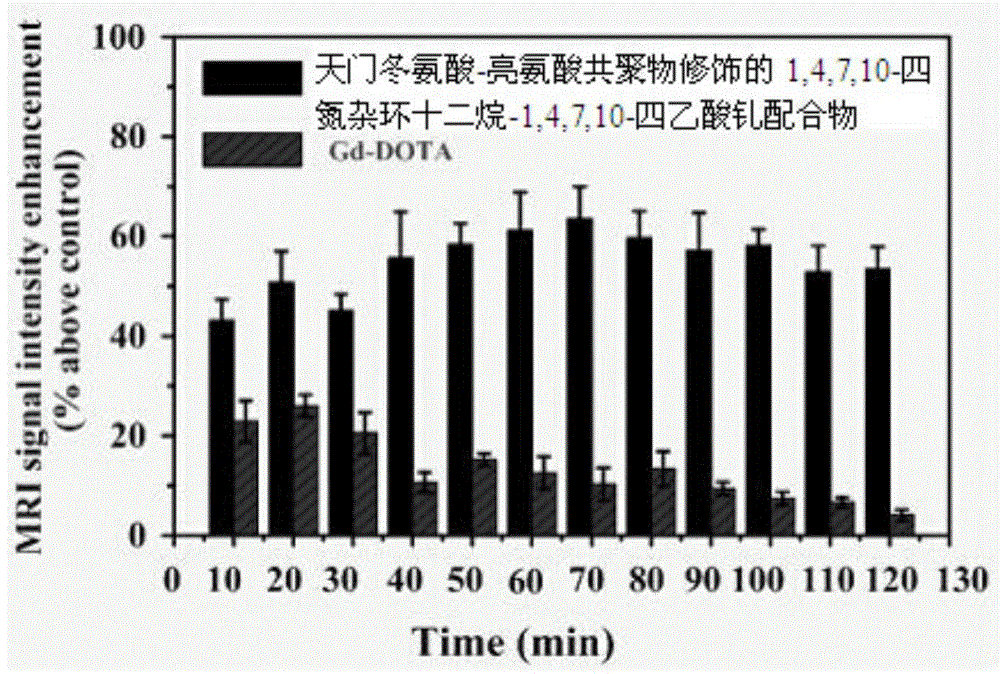
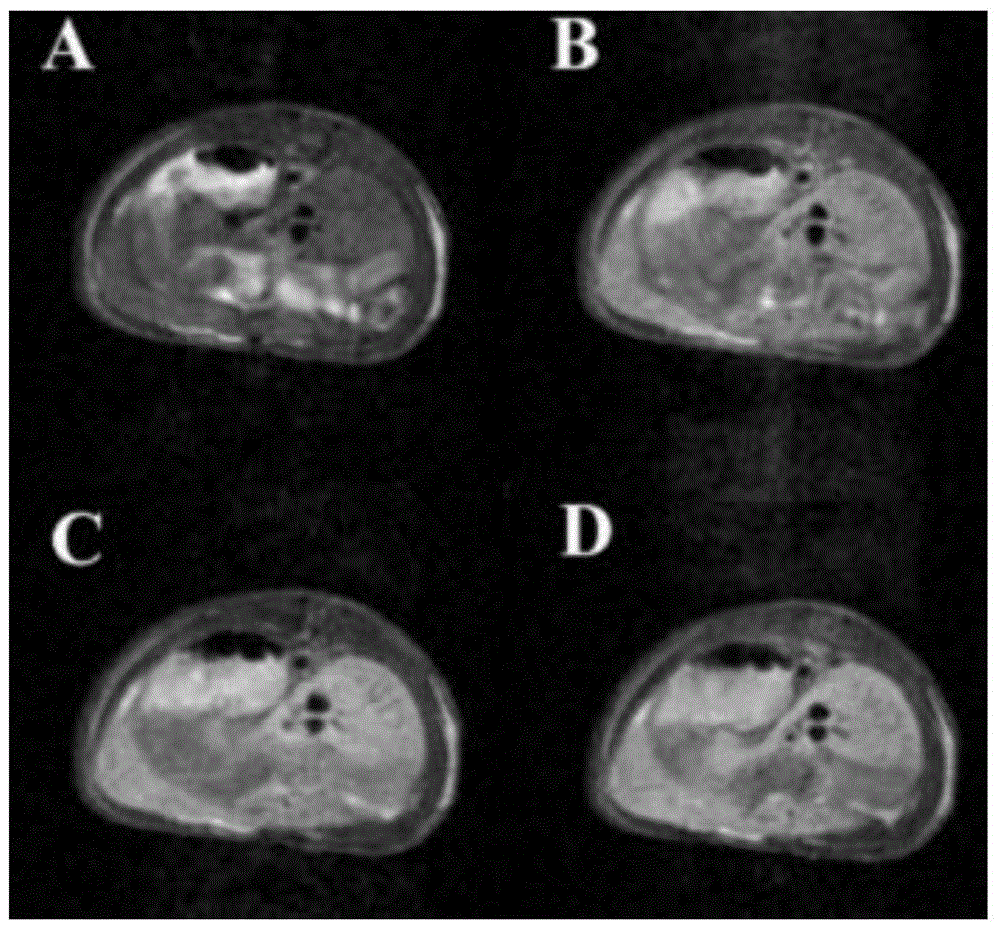
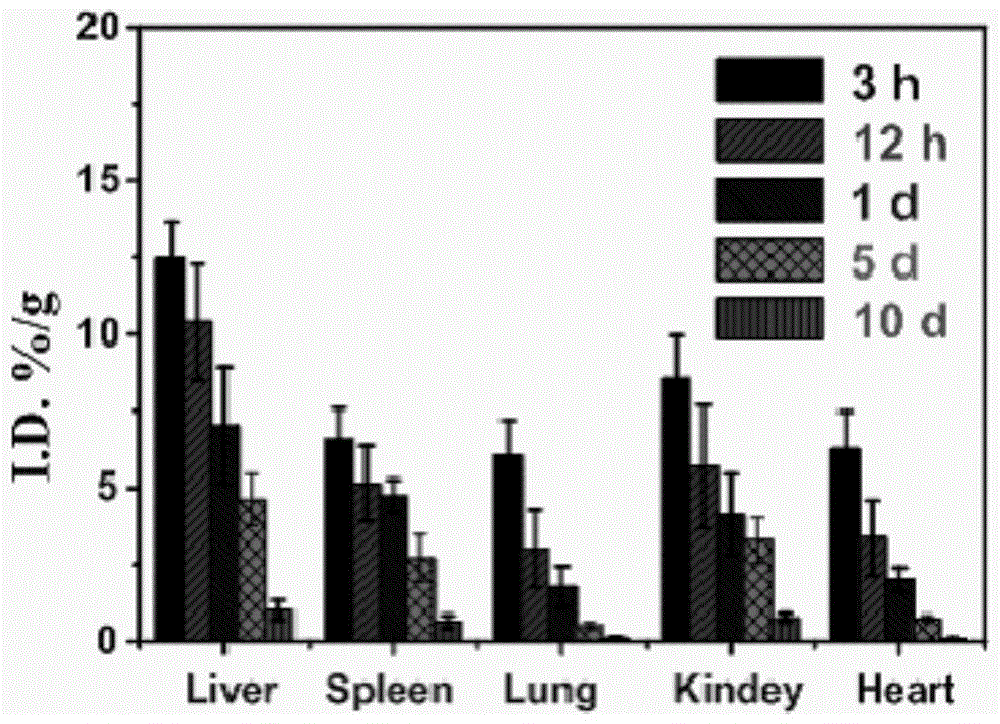
Paramagnetic metal complex modified by aspartic acid-leucine copolymer and its preparation method and application
A technology of leucine copolymer and aspartic acid, applied in preparations for in vivo experiments, pharmaceutical formulations, drug delivery, etc., can solve the problems of low biodegradability, low liver targeting immunity, etc., and achieve Long imaging time, good water solubility, and the effect of improving the level of early diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid gadolinium complex with aspartic acid-leucine copolymer modified by ethylenediamine
[0045] (1) Mix 20 grams of L-aspartic acid with 10 grams of L-leucine, add 10 grams of phosphoric acid solution with a mass fraction of 85% to the mixed mixture and stir evenly, under reduced pressure ( React at 24mmHg) for 5 hours, control the reaction temperature at 165°C, precipitate with deionized water, filter, wash, and dry under reduced pressure to obtain aspartic acid-leucine copolymer;
[0046] (2) Dissolve the aspartic acid-leucine copolymer synthesized in (1) in the N,N-dimethylformamide solution at room temperature, and add ethylenediamine and ethylenediamine dropwise under stirring. The mass ratio to the aspartic acid-leucine copolymer was 2.8:1, stirred at room temperature for 4 hours, ether precipitated, filtered, dialyzed, and lyophilized to obtain the aminated aspartic acid-leucine copolymer;
[0047] (3...
Embodiment 2
[0050] Preparation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid gadolinium complex with aspartic acid-leucine copolymer modified by ethylenediamine
[0051] (1) Mix 2 g of L-aspartic acid with 20 g of L-leucine, add 8 g of phosphoric acid solution with a mass fraction of 85% to the mixed mixture and stir evenly, under reduced pressure ( React at 30mmHg) for 5 hours, control the reaction temperature at 165°C, precipitate with deionized water, filter, wash, and dry under reduced pressure to obtain an aspartic acid-leucine copolymer;
[0052] (2) Dissolve the aspartic acid-leucine copolymer synthesized in (1) in the N,N-dimethylformamide solution at room temperature, and add ethylenediamine and ethylenediamine dropwise under stirring. The mass ratio to the aspartic acid-leucine copolymer was 2.8:1, stirred at room temperature for 4 hours, ether precipitated, filtered, dialyzed, and lyophilized to obtain the aminated aspartic acid-leucine copolymer;
[0053] (3) Dissolve ...
Embodiment 3
[0056] Preparation of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid gadolinium complex with aspartic acid-leucine copolymer modified by ethylenediamine
[0057] (1) Mix 20 g of L-aspartic acid with 20 g of L-leucine, add 15 g of phosphoric acid solution with a mass fraction of 85% to the mixed mixture and stir evenly, under reduced pressure ( React at 200mmHg) for 5 hours, control the reaction temperature at 100°C, precipitate with deionized water, filter, wash, and dry under reduced pressure to obtain aspartic acid-leucine copolymer;
[0058] (2) Dissolve the aspartic acid-leucine copolymer synthesized in (1) in the N,N-dimethylformamide solution at room temperature, and add ethylenediamine and ethylenediamine dropwise under stirring. The mass ratio to the aspartic acid-leucine copolymer was 2.8:1, stirred at room temperature for 4 hours, ether precipitated, filtered, dialyzed, and lyophilized to obtain the aminated aspartic acid-leucine copolymer;
[0059] (3) Dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com