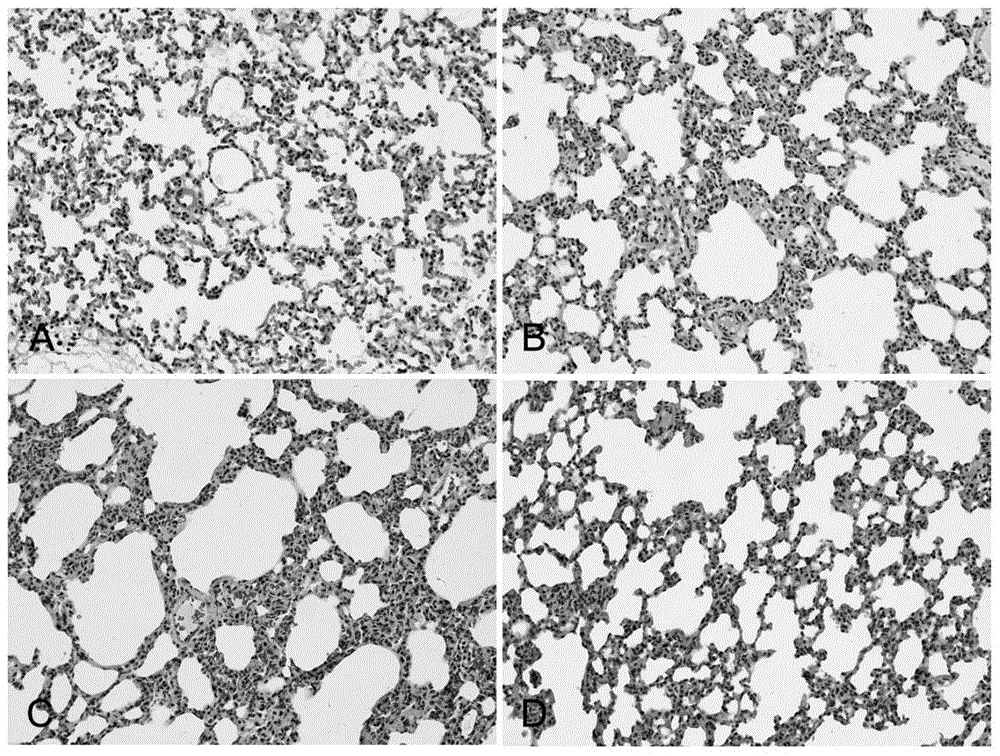
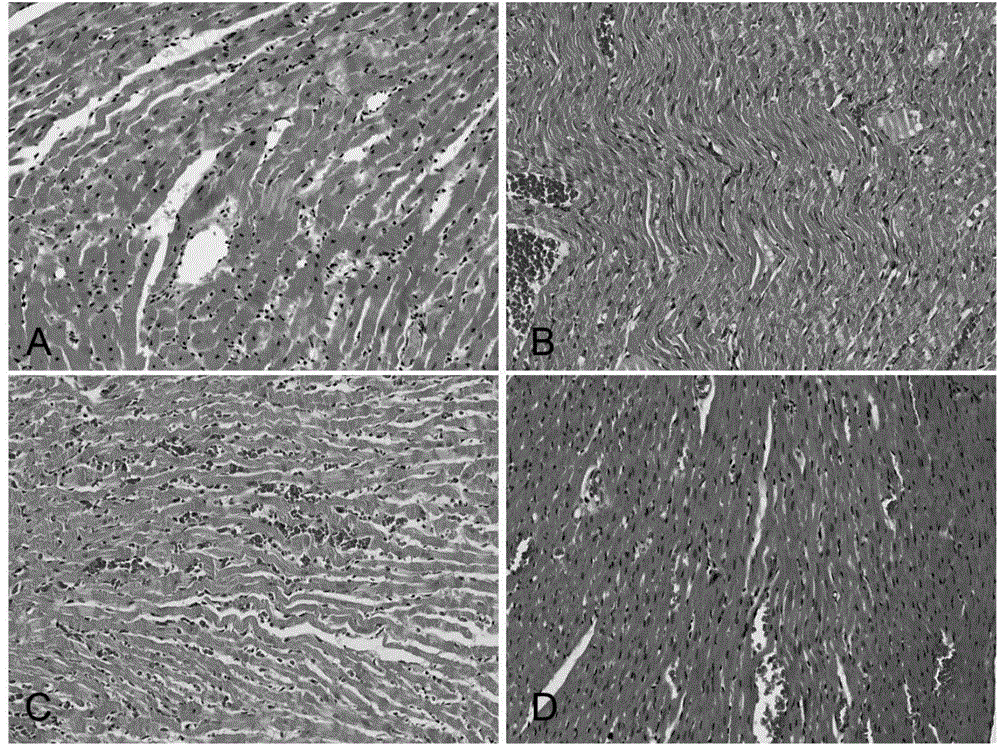
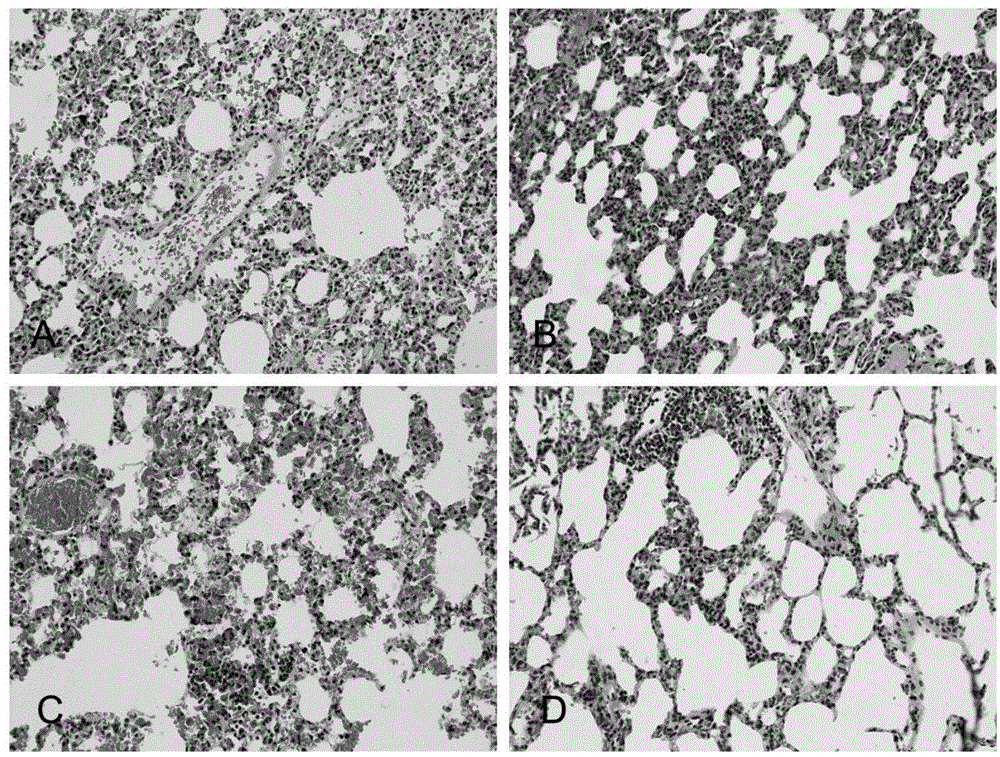
Application of N-acetylcysteine in preparing medicament for preventing decompression sickness caused by fast buoyant ascent escape
A technology of acetylcysteine and decompression sickness, applied in the direction of drug combination, medical formula, respiratory system diseases, etc., can solve problems that have not been used to prevent decompression sickness, and have not been proved by research to prevent decompression sickness, so as to alleviate pathology Change, improve heart function, reduce lung damage and inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Injection drug for experiment: N-acetylcysteine was formulated into a 250 mg / ml solution with physiological saline, and prepared into an injection.
[0021] Experimental animals and grouping: 40 healthy male SD rats, weighing 220-260 g, were purchased from Shanghai Slack Experimental Animal Co., Ltd. SD rats were randomly divided into acetylcysteine 250mg / kg prevention group, acetylcysteine 500mg / kg prevention group, acetylcysteine 1000mg / kg prevention group and normal saline control group, 10 rats in each group.
[0022] Experimental method: 60 minutes before treatment in the prevention group, N-acetylcysteine solution was injected intraperitoneally (injection doses were 250mg / kg, 500mg / kg and 1000mg / kg), and the control group was treated for 60 minutes. Afterwards, the experimental animals were placed in the pressurized chamber for rapid ascending and escaping, and the door was closed, and a self-programmed computer was used to automate the rapid ascending a...
Embodiment 2
[0028] Experimental injection drug: Ashintai (acetylcysteine injection, 20ml, 4g).
[0029] Experimental animals and grouping: 40 healthy male SD rats, weighing 220-260 g, were purchased from Shanghai Slack Experimental Animal Co., Ltd. SD rats were randomly divided into acetylcysteine 150mg / kg prevention group, acetylcysteine 300mg / kg prevention group, acetylcysteine 600mg / kg prevention group and normal saline control group, 10 rats in each group.
[0030] Experimental method: 60 minutes before the treatment of the prevention group, acetylcysteine injection (injection doses were 150mg / kg, 300mg / kg and 600mg / kg) was injected intraperitoneally, and 60 minutes before the treatment of the normal saline control group, the Equal volume of normal saline, and then put the experimental animals in the pressurized chamber for rapid escaping and escape, close the door, and use the self-programmed computer to automate the rapid escaping and escaping procedure, using compressed air...
Embodiment 3
[0034] Injection drug for experiment: use normal saline to make N-acetylcysteine into a 500mg / mL solution, and prepare injection; Ashintai (acetylcysteine injection, 20ml, 4g).
[0035] Experimental animals and grouping: 32 male healthy New Zealand rabbits, weighing 2.3-2.7 kg, were purchased from Shanghai Slack Experimental Animal Co., Ltd. Rabbits were randomly divided into acetylcysteine 300mg / kg prevention group (intraperitoneal injection), intraperitoneal injection control group, Ashintai 400mg / kg prevention group (intravenous injection) and ear vein injection control group, 8 rabbits in each group .
[0036] Experimental method: 30 minutes before the treatment of the acetylcysteine 300mg / kg prevention group, intraperitoneal injection of N-acetylcysteine solution (injection dose is 300mg / kg), 30 minutes before the treatment of the control group, intraperitoneal injection Equal volume of normal saline, Asixintai 400mg / kg prevention group 15 minutes before treatm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com