Vaccine protective agent premixing auxiliary material and preparation method thereof
A technology of vaccine protective agent and premixed auxiliary materials, which is applied in the field of vaccine production technology and biomedicine, can solve the problems such as no reports of vaccine protective agent premixed auxiliary materials, and achieve the effect of convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of sugar solution: Weigh 60g of trehalose, 10g of sucrose, and 10g of lactose respectively, add 200ml of purified water to dissolve, then add 0.4g of activated carbon, stir for 30min, then filter through a 0.22μm filter membrane, and then filter the filtrate through a filter with a molecular cut-off range of 5000-10000d hollow fiber ultrafiltration membrane ultrafilter ultrafiltration.
[0030] Preparation of salt solution: Weigh 8.5g of sodium chloride, 3.5g of disodium hydrogen phosphate dodecahydrate, and 0.25g of sodium dihydrogen phosphate dihydrate, add 50ml of purified water to dissolve, then add 0.1g of activated carbon, stir for 30min, and pass through 0.22 The filter membrane of μm is filtered, and the filtrate is ultrafiltered by an ultrafilter equipped with a hollow fiber ultrafiltration membrane with a molecular cut-off range of 5000-10000d.
[0031] Concentrate and crystallize the sugar solution and salt solution obtained above under the condit...
Embodiment 2
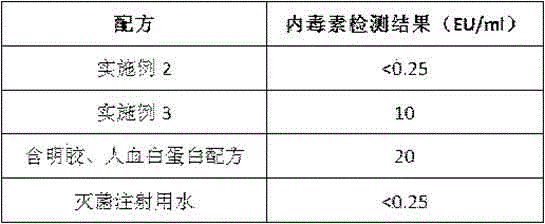
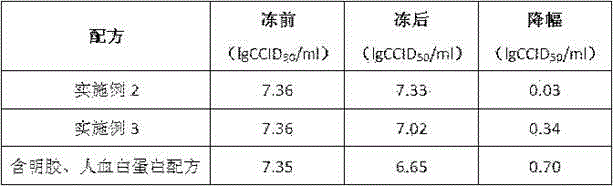
[0034] Take 10 g of the premixed auxiliary material of the vaccine protection agent of Example 1, add 100 ml of water for injection, sterilize by 0.1 um filter, and set aside at 4°C. According to the volume of 0.6ml per person, the virus content is 7.00-7.38 lgCCID 50 / ml, add the stock solution of hepatitis A virus live virus, stir and mix the resulting vaccine mixture and pre-cool at the temperature of -20°C- -50°C for 3-5 hours, and the shelf temperature of the freeze-drying box is -35°C- -50°C , turn on the vacuum, sublimate, and the shelf temperature of the secondary freeze-drying box is 15°C-30°C, and the freeze-dried live attenuated hepatitis A vaccine is made.
Embodiment 3
[0036] After getting and mixing each adjuvant of the same amount as the premixed adjuvant of the vaccine protection agent of Example 1, add 100ml of water for injection, and make the freeze-dried live attenuated hepatitis A vaccine by the method of embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com