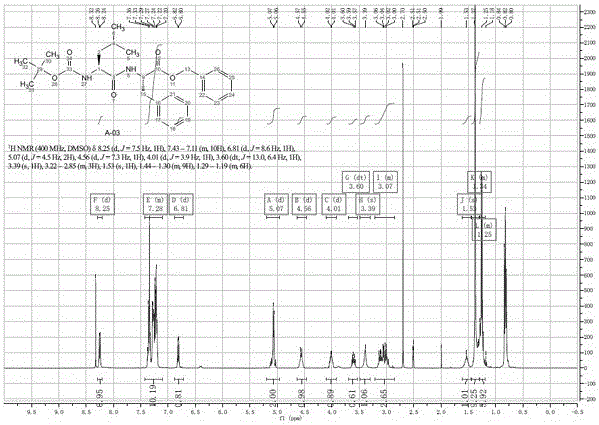
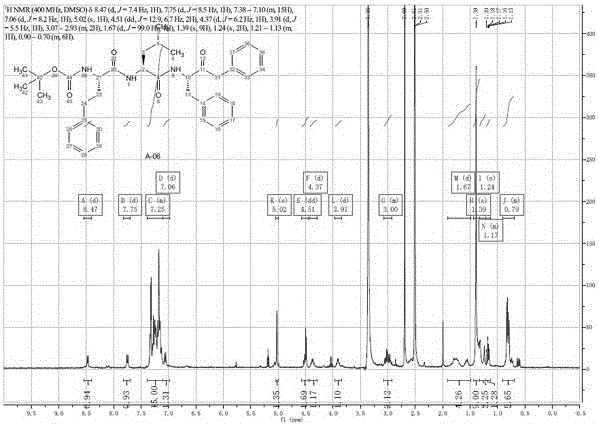
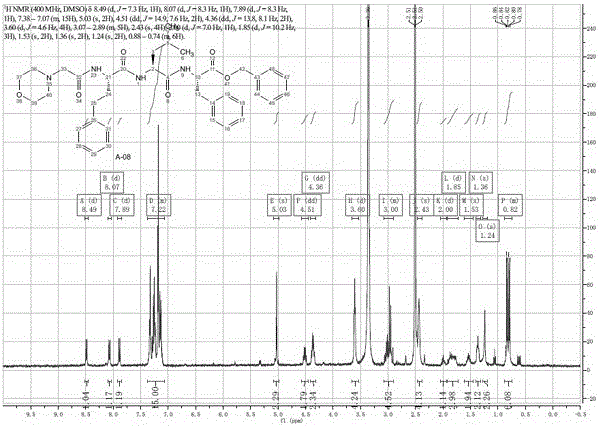
A kind of preparation method of carfilzomib
A technology of carfilzomib and compounds, which is applied in the field of preparation of carfilzomib, can solve the problems of long reaction time between PyBOP and HOBT, difficult removal of by-products, high temperature control requirements, etc., and achieve shortened preparation time, simple feeding and high reaction short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Compound A is prepared by the following steps: adding sodium hydroxide solution (wherein the content of sodium hydroxide is 0.38mol, The content of water is 200mL), then add 0.18mol of Boc anhydride, react at 25°C for 22h, then add acid under ice-water bath to pH 1, extract three times with ethyl acetate to obtain the organic phase, wash the organic phase twice with saturated saline , then dried to remove water, filtered to retain the filtrate, and the filtrate was spin-dried to obtain 40 g of a colorless viscous liquid, which became a paste after cooling, which was Compound A.
[0038] Compound B was prepared by the following steps: put 0.3mol of L-phenylalanine, 1.51mol of benzyl alcohol, 0.36mol of p-toluenesulfonic acid and 700mL of toluene into the reaction flask in sequence, raise the temperature to reflux, maintain reflux, and divide the water React until the reaction is complete (12 h), lower to 25°C, concentrate until solids are precipitated, add 400 mL ethyl a...
Embodiment 2
[0049] Compound A is prepared by the following steps: adding sodium hydroxide solution (wherein the content of sodium hydroxide is 0.30mol, The content of water is 200mL), then add 0.15mol of Boc anhydride, react at 20°C for 20h, then add acid in an ice-water bath until the pH is 2, extract with ethyl acetate three times to obtain the organic phase, wash the organic phase with saturated saline twice , and then dried to remove water, filtered to retain the filtrate, and the filtrate was spin-dried to obtain 35 g of a colorless viscous liquid, which became a paste after cooling, which was Compound A.
[0050] Compound B was prepared by the following steps: 0.3 mol of L-phenylalanine, 1.2 mol of benzyl alcohol, 0.3 mol of p-toluenesulfonic acid and 700 mL of toluene were sequentially put into the reaction flask, heated to reflux, kept reflux, and separated water React until the reaction is complete (10h), lower to 20°C, concentrate until solid precipitates, add 400 mL of ethyl ac...
Embodiment 3
[0061] Compound A is prepared by the following steps: adding sodium hydroxide solution (wherein the content of sodium hydroxide is 0.45mol, The content of water is 200mL), then add 0.225mol of Boc anhydride, react at 30°C for 24h, then add acid in ice-water bath until the pH is 0, extract three times with ethyl acetate to obtain the organic phase, and wash the organic phase twice with saturated saline , and then dried to remove water, filtered to retain the filtrate, and the filtrate was spin-dried to 39 g of a colorless viscous liquid, which became a paste after cooling, which was compound A.
[0062] Compound B was prepared by the following steps: put 0.3mol of L-phenylalanine, 1.8mol of benzyl alcohol, 0.45mol of p-toluenesulfonic acid and 700mL of toluene into the reaction flask in sequence, raise the temperature to reflux, keep reflux, and divide the water React until the reaction is complete (14h), lower to 30°C, concentrate until solid precipitates, add 400 mL of ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com