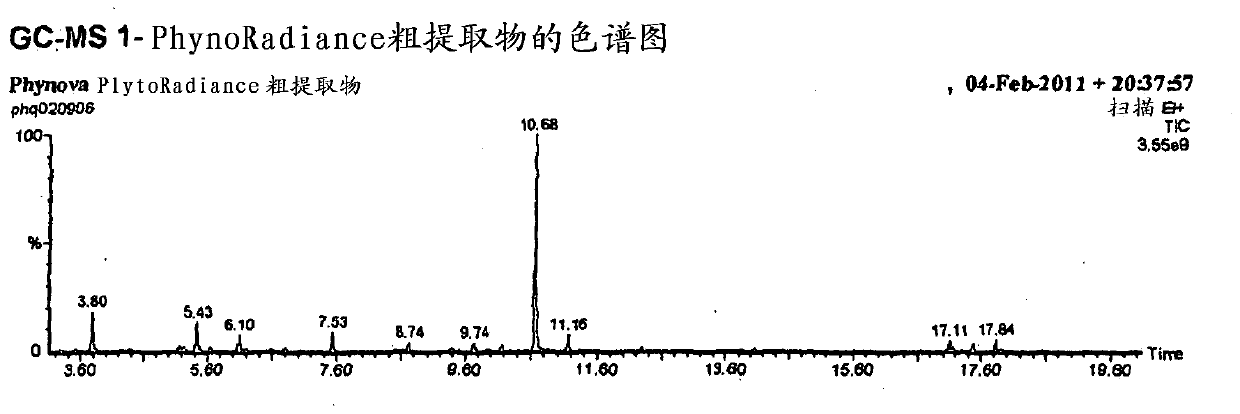
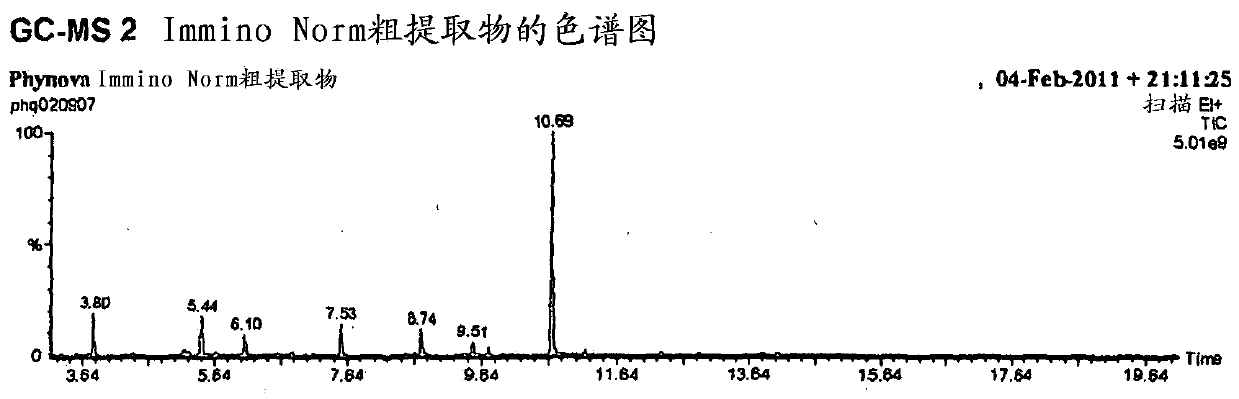
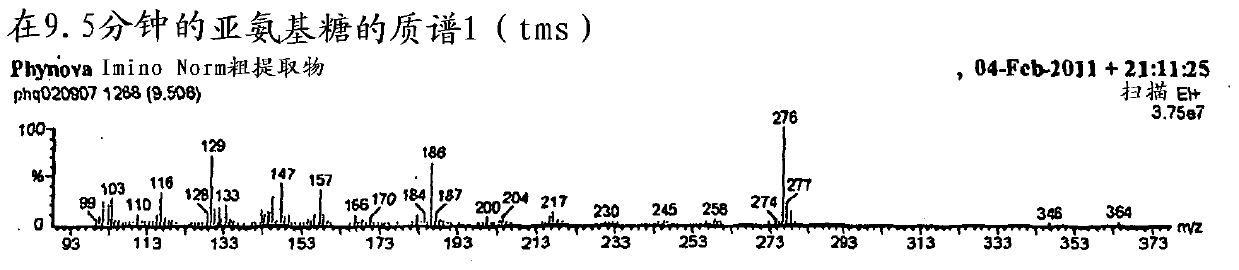
Mulberry extract rich in n-acids of imino sugars and/or 2-pipericollic acids
一种氨基酸类、提取物的技术,应用在含有效成分的医用配制品、植物/藻类/真菌/苔藓成分、药物组合等方向,能够解决副作用等问题,达到降低副作用、改善生物利用度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] starting extract
[0142] Dry extract of mulberry leaves, batch ML091210
[0143] method
[0144] The sample was dissolved in water, and exchanged by strongly acidic cation (IR120H in column 2×30cm + Form resin) fractional distillation, get
[0145] i. Water-shifted unretained samples (expected to contain mostly sugars, flavonoids, etc.);
[0146] ii. 2M pyridine-shifted bound fraction (typically removes residual sugars, phenols, and neutral and acidic amino acids); and
[0147] iii. Bound fraction transferred from 2M ammonia solution (expected to contain any alkaloids [imino sugars] and basic amino acids)
[0148] Some DNJ was detected in the unretained material (perhaps due to sample overloading or pH), so it was passed through a second IR120 column (same size) to obtain a new unretained sample and a second ammonia fraction . The second unretained sample did not contain DNJ.
[0149] The fractions are listed in Table 1 below:
[0150]
[0151] This fraction...
Embodiment 2
[0183] The extract of Example 1 was fractionated into four fractions using cation exchange chromatography (IR120H+ form).
[0184] The unretained fraction contained sugars with imino sugars transferred with pyridine and then ammonia.
[0185] Some DNJ was present in each retained fraction, but most was present in the first ammonia fraction. Other imino sugars were also tested, including experimental mulberry alkaloids, DAB, certain hydroxylated imino acids, and other possible new imino sugars.
[0186] Table 4 below shows the enzyme inhibition observed by the fractions in Example 1.
[0187]
[0188]
[0189] In embodiment 2, purpose is:
[0190] a. Further decompose the components in all four fractions:
[0191] b. Analyze the compound by GC-MS and 500MHz NMR, identifying as many components as possible; and
[0192] c. Glycosidase assays were performed on the purified compounds to further discern the inhibitory effects conferred by the different components.
[0193] ...
Embodiment 3
[0246] The fractions described in Example 2 were analyzed to obtain additional information about the iminosugars and sugars of the samples.
[0247] The sugar fraction accounted for almost half of the samples and showed activity against various glycosidases and glycogen phosphorylases.
[0248] Sugars such as sucrose, trehalose and glucose do not significantly inhibit these enzymes.
[0249] In this example, an attempt was made to characterize the active components of the sugar fraction and further observe its activity.
[0250] At this point, it is further fractionated using a set of ion exchange columns.
[0251] method
[0252] The IR120 unretained fraction from Example 1 was placed on anion exchange resin CG400 (OH - Chromatographic analysis on (1-11) and two acetic acid fractions (Table 5 - Example 2) were obtained. Still in this example these fractions were further fractionated and the activity of said fractions was investigated further.
[0253] analyze
[0254] G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com