Pharmaceutical composition of recombinant glucagon-like peptide-1 receptor stimulant for injection
A technology for glucagon and receptor agonists, which is applied in the field of pharmaceutical compositions for injection of recombinant glucagon-like peptide-1 receptor agonists, and can solve problems affecting the stability of preparations, precipitation and precipitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
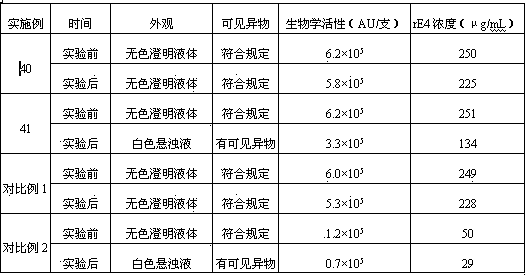
Embodiment 1
[0104] Embodiment 1: Preparation of rE4 freeze-dried powder injection
[0105] Component Weight (g)
[0106] Recombinant glucagon-like peptide-1 receptor agonist 0.12
[0107] Citric acid 2.45
[0108] Sodium citrate 2.36
[0109] Mannitol 30
[0110] ① Weigh citric acid, sodium citrate, and mannitol according to the above prescription quantities, and add 800mL of water for injection to pre-dissolve;
[0111] ② Add appropriate amount of rE4 stock solution;
[0112] ③ Dilute the water for injection to 1000mL, the pH of the solution is 4.5, and the concentration of citric acid-sodium citrate buffer salt is 20mM;
[0113] ④ Sterilize and filter with a 0.22 μm filter membrane;
[0114] ⑤Aliquot 1mL / cartridge and freeze-dry.
Embodiment 2
[0115] Embodiment 2: Preparation of rE4 injection
[0116] Component Weight (g)
[0117] Recombinant glucagon-like peptide-1 receptor agonist 0.25
[0118] Citric acid 1.16
[0119] Sodium citrate 4.26
[0120] Mannitol 30
[0121] Phenol 2.5
[0122] ① Weigh citric acid, sodium citrate, mannitol, and phenol according to the above prescription quantities, and add 800mL of water for injection to pre-dissolve;
[0123] ② Add appropriate amount of rE4 stock solution;
[0124] ③ Dilute the water for injection to 1000mL, the pH of the solution is 5.6, and the concentration of citric acid-sodium citrate buffer salt is 20mM;
[0125] ④ Sterilize and filter with a 0.22 μm filter membrane;
[0126] ⑤ Dispense according to 1.2mL / cartridge.
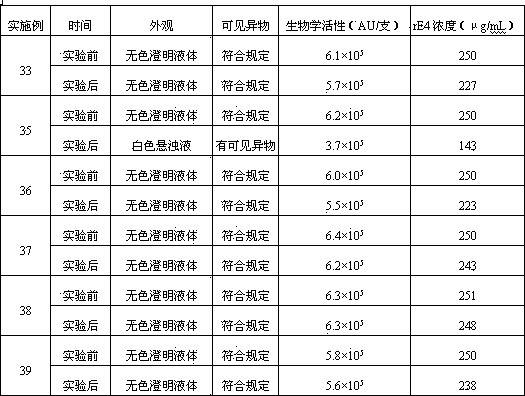
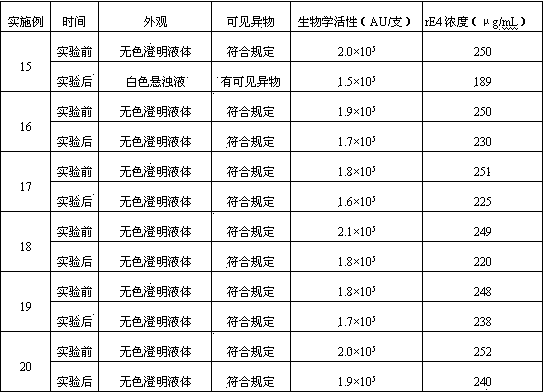
Embodiment 3~7
[0127] Embodiment 3~7: the investigation of rE4 concentration
[0128] Referring to the prescription and preparation process of Example 1, only the specifications of the freeze-dried powder injection were changed to 0.03, 0.05, 0.10, 0.15, 0.20 mg / bottle respectively, and the freeze-dried powder injection was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com