A kind of preparation method of 2-(tert-butylamino) carbonyl pyridine compound
A technology of tert-butylamino and carbonylpyridine, which is applied in the field of preparation of 2-(tert-butylamino)carbonylpyridine compounds, can solve the problems of complicated operation, reduced yield, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
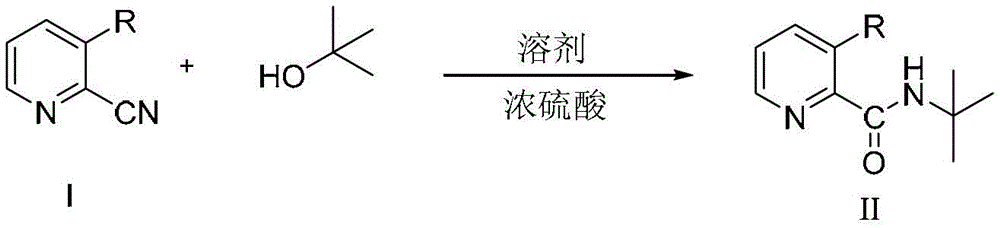
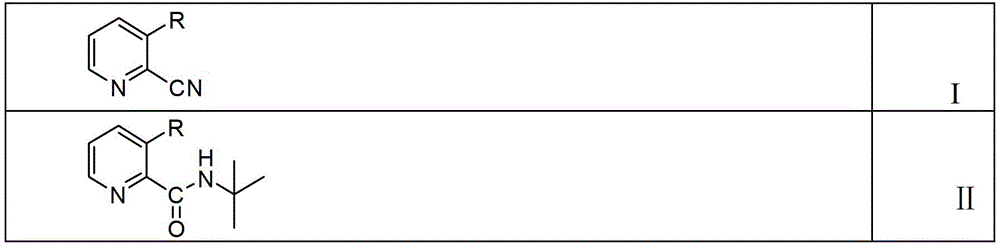
[0022] Specifically, the preparation method of the 2-(tert-butylamino)carbonylpyridine compound with the structure shown in the formula II provided by the present invention comprises steps:
[0023] In the first step, a 2-cyanopyridine compound having a structure as shown in formula I, tert-butanol and a solvent are mixed to obtain a reaction solution 1;
[0024] In the second step, concentrated sulfuric acid is added dropwise to the reaction solution 1, and then the temperature is raised to 60°C-the reflux temperature of the solvent for reaction to obtain the reaction solution 2;
[0025] In the third step, the reaction solution 2 and water were mixed, and then the aqueous phase was adjusted to pH=9-10 with concentrated ammonia water to obtain 2-(tert-butylamino)carbonylpyridine compounds with the structure shown in formula II.
[0026] In the above first step, the solvent is selected from formic acid, acetic acid or a mixture thereof.
[0027] In the above first step, the m...
Embodiment 1
[0045] Preparation of 3-methyl-2-(tert-butylamino)carbonylpyridine
[0046]Add 2-cyano-3-picoline (5.00g, 42.3mmol), tert-butanol (13.0ml, 138mmol), formic acid (19.9ml, 517mmol), glacial acetic acid (7.5ml, 130mmol) into the dry reactor , stirred at room temperature for 5 minutes. Then, concentrated sulfuric acid (2.0ml, 39.6mmol) was slowly added dropwise to the reaction solution, and the reaction was completed within 30 minutes, and then the temperature was gradually raised to 60°C. After the reaction, the reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, 20.0ml of water was added to the residue to dissolve, and then the aqueous phase was adjusted to pH=9-10 with 15.0ml of concentrated ammonia water. Extracted with toluene, and evaporated the solvent under reduced pressure to obtain 8.37 g of crystals, with a yield of 98.64% and a GC purity of 95.90%. Recrystallized from petroleum ether to obtain 6.47g of white crystals, ...
Embodiment 2
[0048] Preparation of 3-methyl-2-(tert-butylamino)carbonylpyridine
[0049] Add 2-cyano-3-picoline (5.00g, 42.3mmol), tert-butanol (17.5ml, 186mmol), formic acid (15.0ml, 390mmol), glacial acetic acid (7.5ml, 130mmol) into the dry reactor , stirred at room temperature for 5 minutes. Then, concentrated sulfuric acid (2.0ml, 39.6mmol) was slowly added dropwise to the reaction solution, and the reaction was completed within 30 minutes, and then the temperature was gradually raised to 60°C. After the reaction, the reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, 20.0ml of water was added to the residue to dissolve, and then the aqueous phase was adjusted to pH=9-10 with 15.0ml of concentrated ammonia water. Extracted with toluene, and evaporated the solvent under reduced pressure to obtain 8.12 g of crystals, yield 95.80%, GC purity 96.00% [GC normalization method: chromatographic column (5%) phenyl-(95%) methylpolysiloxane Alk...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com