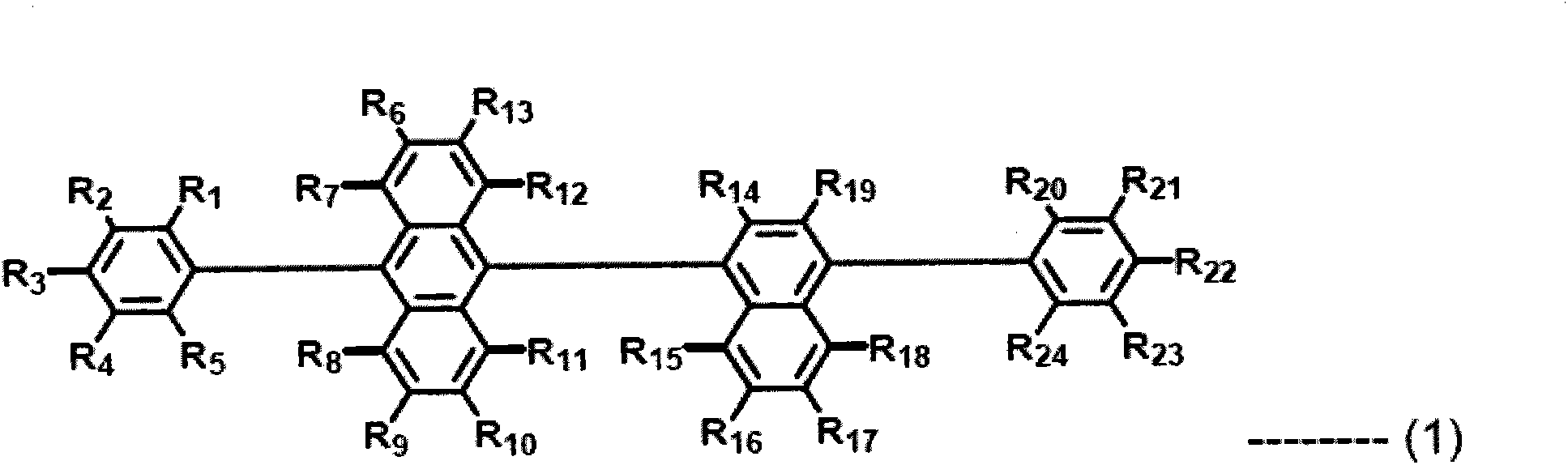
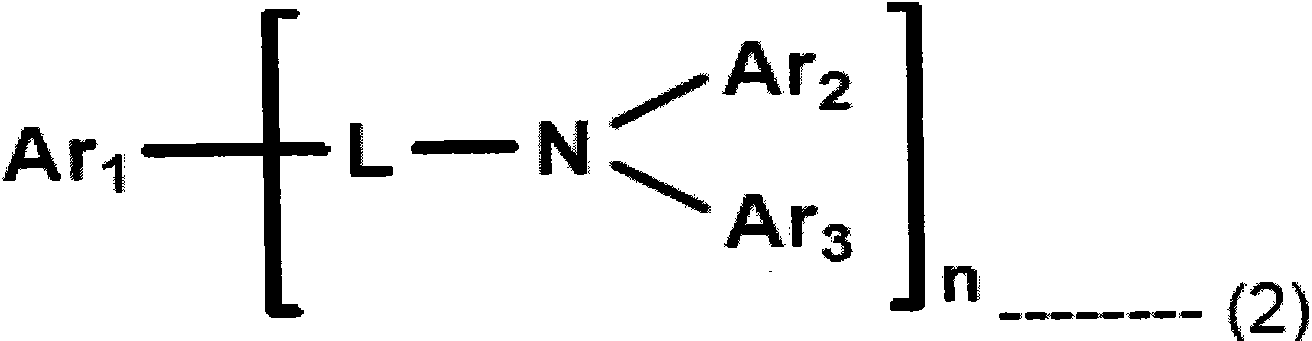
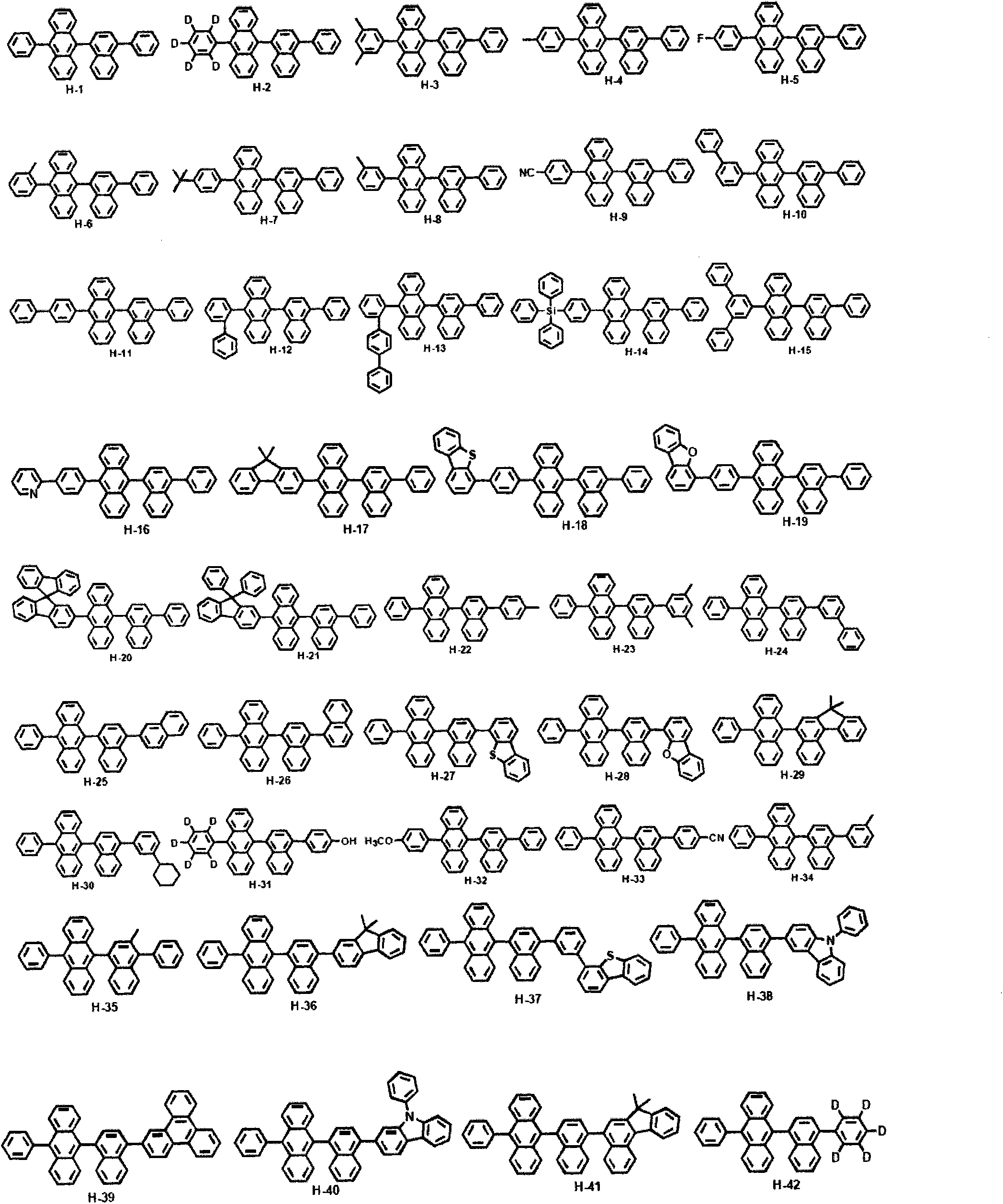Composition including dopant and matrix, and organic electroluminescent device
A heteroaryl and aryl technology, which is applied in the combination of dopants and hosts and in the field of organic electroluminescent devices, can solve the problems of difficulty in obtaining a blue light-emitting layer, unsatisfactory luminous efficiency, and unsatisfactory operating life of power efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of compound H-1
[0062]
[0063] Preparation of Compound 1-1
[0064] Mix 1,4-dibromonaphthalene (60 g, 0.20 mol), phenylboronic acid (30 g, 0.24 mol), Pd(PPh 3 ) 4(9.6 g, 8.3 mmol) and Na 2 CO 3 (66 grams, 0.60 moles), add 1200 milliliters of toluene afterwards, 300 milliliters of ethanols, and H 2 O 300 mL to dissolve the mixture, and the mixture was stirred at 100 °C for 12 h. After the reaction, the H 2 O was slowly added to the mixture to complete the reaction, the organic layer was extracted with ethyl acetate, and MgSO 4 Remove residual moisture. The residue was then dried and separated using a column to obtain compound 1-1 (26 g, 43%).
[0065] Preparation of Compound H-1
[0066] In mixed compound 1-1 (26 g, 0.09 mol), 10-phenylanthracene-9-ylboronic acid (30 g, 0.11 mol), Pd(PPh 3 ) 4 (6.3 g, 5.51 mmol) and K 2 CO 3 (38 grams, 0.30 moles), add 280 milliliters of toluene, 140 milliliters of ethanol, and H 2 1...
Embodiment 2
[0067] Embodiment 2: the preparation of compound H-42
[0068]
[0069] Preparation of compound 2-1
[0070] Compound 2-1 was prepared by the same synthetic method as Compound 1-1 except for using 1,4-dibromonaphthalene and dS-phenylboronic acid.
[0071] Preparation of Compound H-42
[0072] Using compound 2-1 and 10-phenylanthracene-9-ylboronic acid compound, compound H-42 (5 g, 52%) was prepared by the same synthetic method as compound H-1.
Embodiment 3
[0073] Embodiment 3: the preparation of compound D-3
[0074]
[0075] In a flask mix 5,9-dibromo-7,7-dimethyl-7H-benzo[c]fluorene (7 g, 0.0174 mol), diphenylamine (7.3 g, 0.0435 mol), palladium acetate (195 mg , 0.87 mmol), tri-tert-butylphosphine (0.8 ml, 1.7 mmol), and NaOt-Bu (5 g, 0.0522 mol), 120 ml of toluene was added to the mixture, and stirred and refluxed at 120 ° C for 3 hours . After the reaction was complete, the organic layer was extracted with ethyl acetate and washed with MgSO 4 Remove residual moisture. The residue was then dried and separated using a chromatography column to obtain compound D-3 (6 g, 55%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com