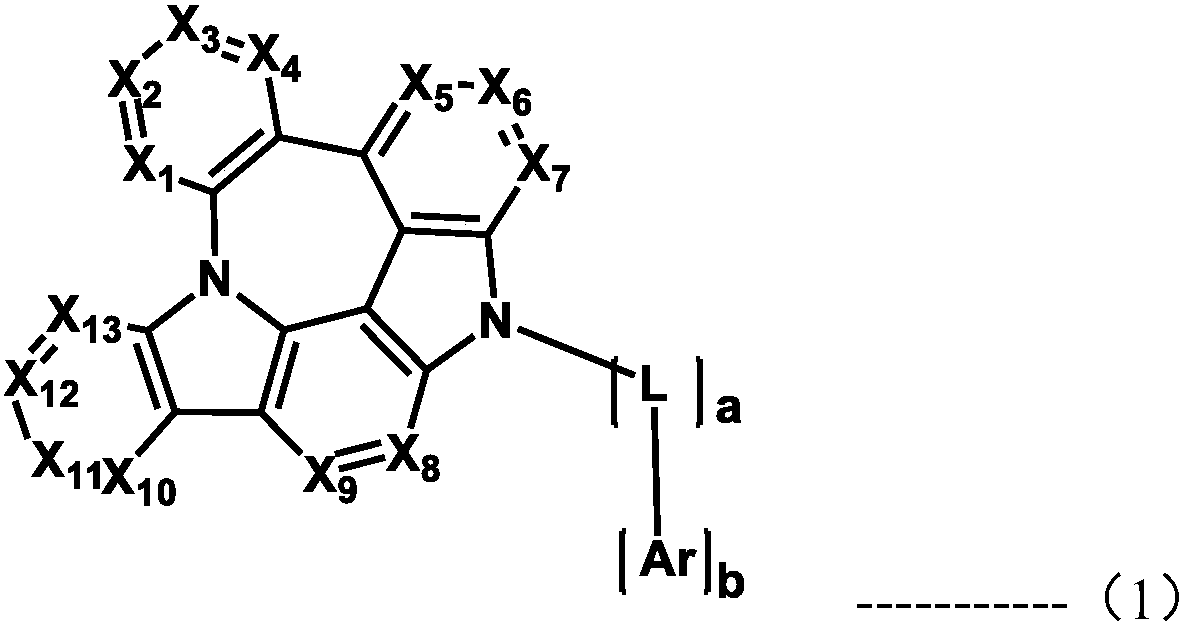
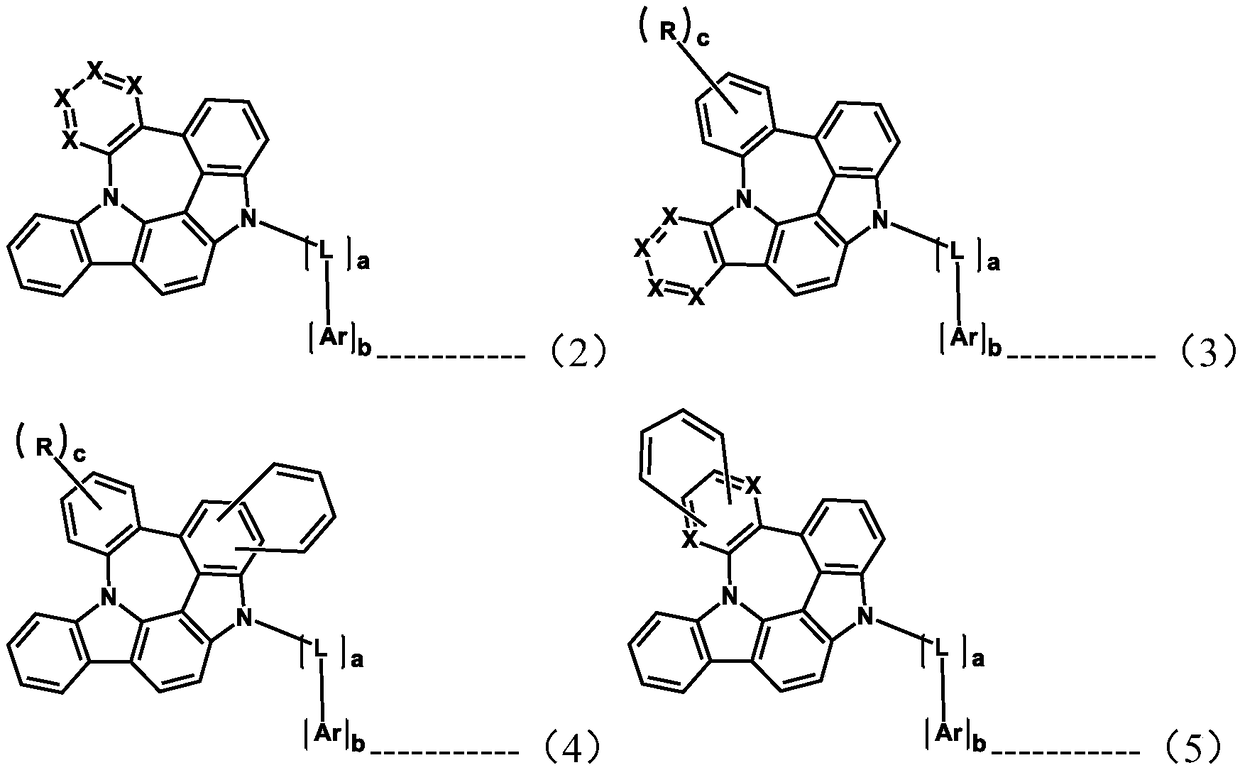
Organic electroluminescent compound and organic electroluminescent device comprising the same
一种化合物、致发光的技术,应用在电致发光光源、有机化学、电光源等方向,能够解决低玻璃化转变温度、有机电致发光装置工作寿命短、没有具体公开等问题,达到低驱动电压的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0117] Example 1: Preparation of Compound C-50
[0118]
[0119] Preparation of compound 1-1
[0120] 36g compound A (125.38mmol), 27g 3-bromo-2-chloro-nitrobenzene (113.98mmol), 4g tetrakis (triphenylphosphine) palladium (3.42mmol), 30g sodium carbonate (284.95mmol), 570mL toluene , 140 mL of ethanol, and 140 mL of distilled water were introduced into the reaction vessel, and the mixture was stirred at 120° C. for 3 hours. After the reaction was completed, the mixture was cooled to room temperature, and extracted with ethyl acetate. The extracted organic layer was then dried with magnesium sulfate, and the solvent was removed using a rotary evaporator. After that, the resulting product was purified by column chromatography to obtain 30 g of Compound 1-1 (yield: 66%).
[0121] Preparation of compound 1-2
[0122] 27g of compound 1-1 (68.20mmol), 1.5g of palladium (II) acetate (6.82mmol), 5.0g of tricyclohexylphosphonium tetrafluoroborate (13.64mmol), 66g of cesium...
example 2
[0128] Example 2: Preparation of Compound C-51
[0129]
[0130]5g compound 1-3 (15mmol), 8.6g 2-(4-bromonaphthalen-1-yl)-4,6-diphenyl-1,3,5-triazine (20mmol), 0.4g palladium acetate (II) (2 mmol), 1.2 g of soil inorganic phosphorus (3 mmol), 2.2 g of sodium tert-butoxide (23 mmol), and 76 mL of o-xylene were introduced into a reaction vessel, and the mixture was stirred under reflux for 3 hours. After the reaction was completed, the mixture was washed with distilled water, extracted with ethyl acetate, and the extracted organic layer was dried with magnesium sulfate. The solvent was removed with a rotary evaporator, and the resulting product was purified by column chromatography to obtain 3.3 g of Compound C-51 (yield: 32%).
[0131]
example 3
[0132] Example 3: Preparation of Compound C-49
[0133]
[0134] 6g compound 1-3 (18mmol), 8.5g 2-(4-bromophenyl)-4,6-diphenyl-1,3,5-triazine (22mmol), 0.4g palladium (II) acetate ( 2 mmol), 1.5 g of soil inorganic phosphorus (4 mmol), 2.6 g of sodium tert-butoxide (27 mmol), and 91 mL of o-xylene were introduced into the reaction vessel, and the mixture was stirred under reflux for 2 hours. After the reaction was completed, the mixture was washed with distilled water, extracted with ethyl acetate, and the extracted organic layer was dried with magnesium sulfate. The solvent was removed with a rotary evaporator, and the resulting product was purified by column chromatography to obtain 10 g of compound C-49 (yield: 86%).
[0135]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com