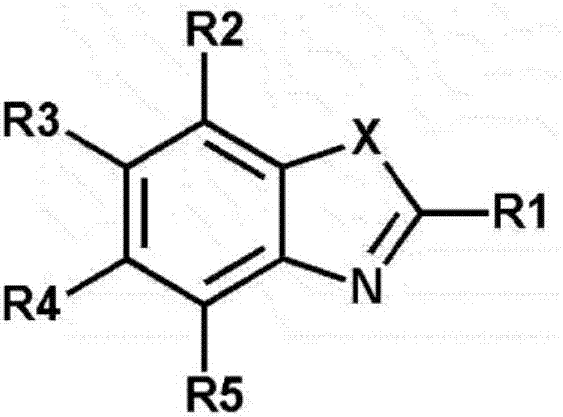
Organic electroluminescent element
A technology of organic light-emitting devices and organic material layers, applied in electrical components, luminescent materials, organic chemistry, etc., can solve the problems of low overall light efficiency of organic light-emitting devices, improve luminous efficiency and color purity, enhance life characteristics, low The effect of drive voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example A
[0225] Synthesis of the following chemical formula A
[0226] [chemical formula A]
[0227]
[0228] In the 2-(4-bromophenyl)-benzo Azole (5g, 18.2mmol), bis(pinacol)diboron (5.1g, 20.1mmol) and potassium acetate (5.4g, 54.7mmol) were introduced into 1,4-dioxane (182ml, 0.1M) and after suspending and stirring the resultant, Pd(dppf)Cl was added thereto 2 (260 mg, 0.36 mmol), and the resultant was heated and stirred at 100° C. for 8 hours. After cooling the reaction solution to room temperature, H was added thereto 2 O (100ml), the resultant was stirred for 10 minutes, then extracted with THF. Remove the aqueous layer and wash with magnesium sulfate (MgSO 4 ) to treat the organic layer, then concentrate. The resultant was crystallized with ethanol (150ml), and then filtered to obtain the compound of chemical formula A (5.3g, yield 90%).
[0229] MS: [M+H] + =322
preparation example B
[0230] Synthesis of the following chemical formula B
[0231] [chemical formula B]
[0232]
[0233] The compound of formula B was synthesized in the same manner as the compound of formula A except that 2-(4-bromophenyl)-benzothiazole was used instead of 2-(4-bromophenyl)-benzo azole.
[0234] MS: [M+H] + =338
preparation example 1
[0235] Synthesis of Chemical Formula 1-4
[0236]
[0237] After the compound of chemical formula A (10g, 31.1mmol) and 1-bromo-3,5-diphenylbenzene (9.6g, 31.1mmol) were dissolved in THF (310ml), dissolved in H 2 K in O (100ml) 2 CO 3 (8.6 g, 62.3 mmol) was suspended and stirred, then tetrakis(triphenylphosphine)palladium(0) (720 mg, 0.62 mmol) was added thereto, and the resultant was refluxed for 8 hours. After the reaction was completed, the temperature was lowered to room temperature, the aqueous layer was removed, and the solution was washed with magnesium sulfate (MgSO 4 ) to treat the organic layer, then filter. The solution was concentrated in vacuo under reduced pressure, and subjected to column purification at a ratio of THF / hexane=1 / 3 to obtain the compound of Chemical Formula 1-4 (10 g, yield: 76%).
[0238] MS: [M+H] + =424
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com