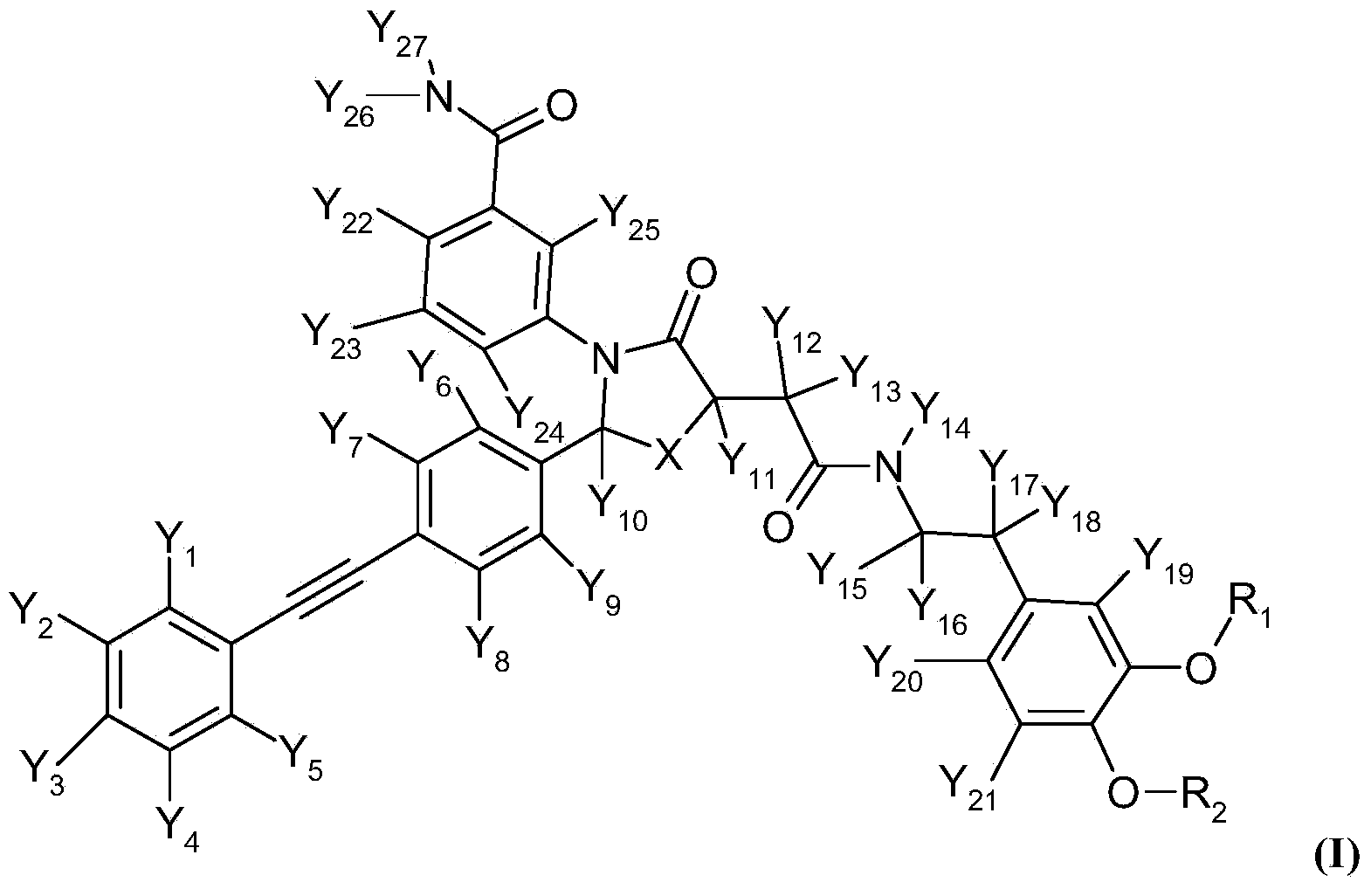
Deuterated thiazolidinone analogues as agonists for follicle stimulating hormone receptor
A technology of deuterated thiazolidinone and solvate, applied in the field of deuterated thiazolidinone analogs, can solve problems such as not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
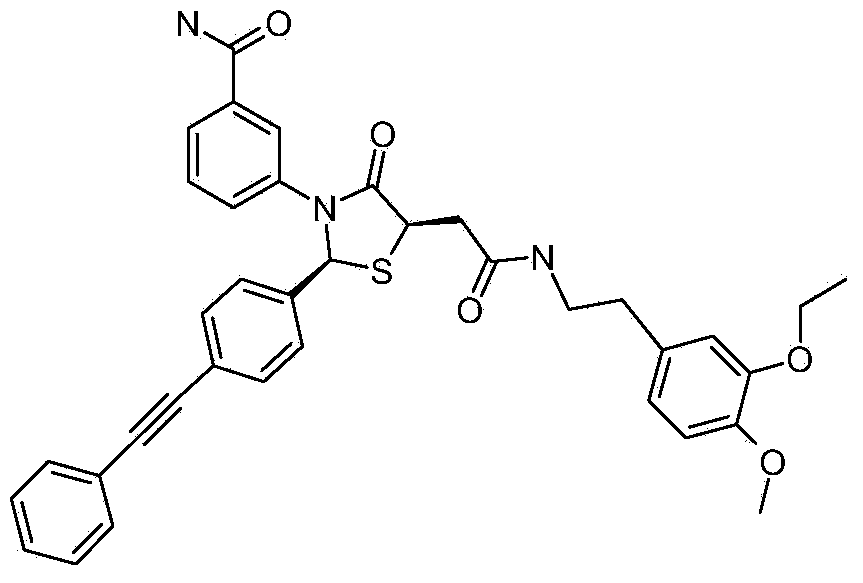
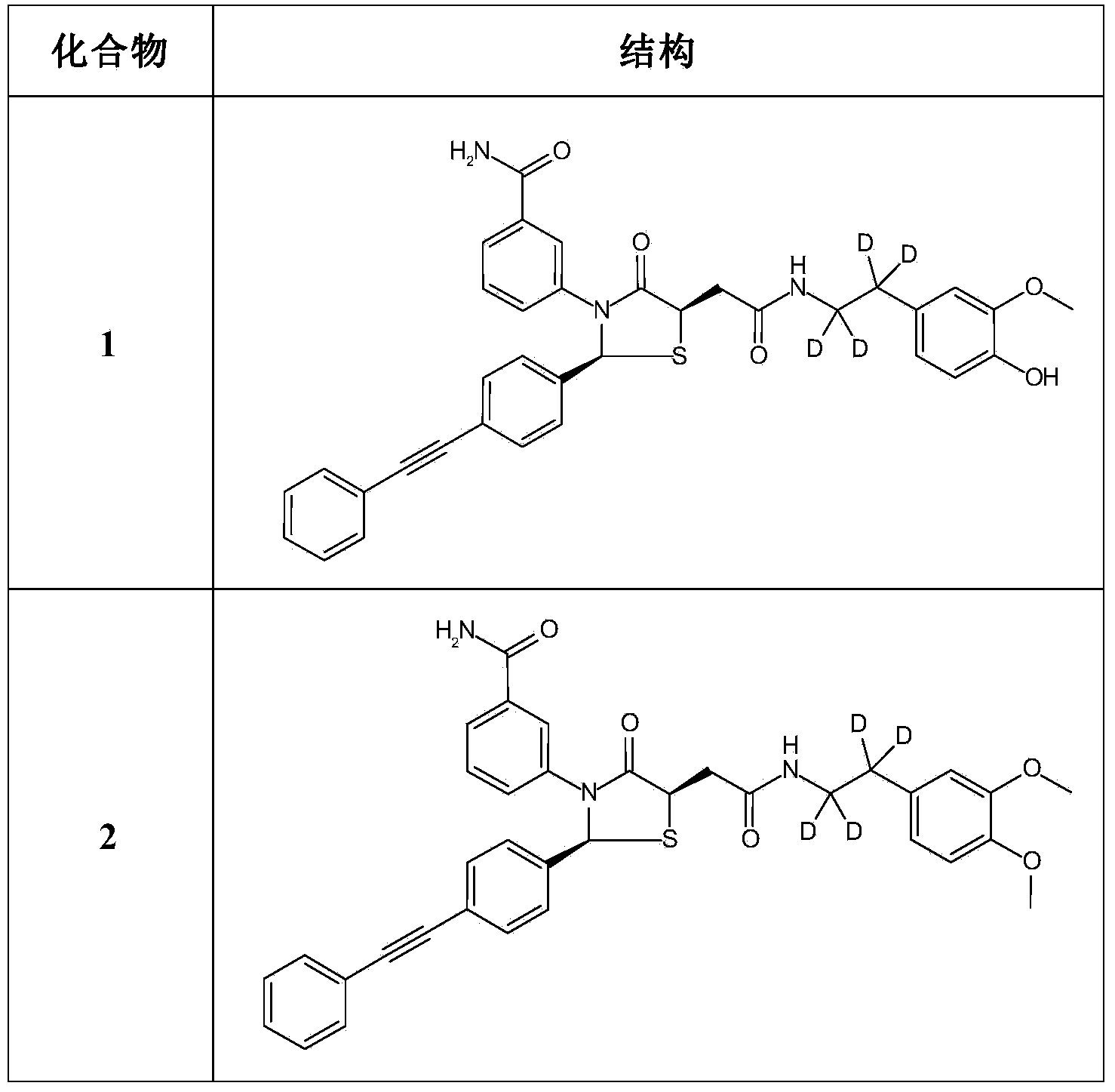
[0161] Example 1: 3-((2S,5R)-4-oxo-5-(2-oxo-2-((1,1,2,2-d 4 -2-(4-Hydroxy-3-methoxyphenyl)ethyl)amino)ethyl)-2-(4-(phenylethynyl)phenyl)thiazolidin-3-yl)benzamide
[0162]
[0163] step 1:
[0164]
[0165] Reference is made to the methods described in WO02 / 09705 and WO02 / 09706.
[0166] To a solution of 4-(phenylethynyl)benzaldehyde (2.8g, 13.6mmol, 1eq) in acetonitrile (240ml) was added (S)-2-mercaptosuccinic acid (6.12g, 40.8mmol, 3eq) and 3 - Aminobenzamide (1.85 g, 13.6 mmol, 1 eq). The reaction was stirred at 83°C for 3 days. The reaction was cooled and filtered to give a solid. with saturated Na 2 CO 3 The solution adjusted the solid to pH = 8-9, and extracted with ethyl acetate (50ml X3). The aqueous phase was adjusted to pH = 3-4 with 4N HCl, and filtered to obtain a solid. The solid was washed with ethanol (10ml) and acetonitrile (10ml) to give the title compound as a pale yellow solid (3g, 51.7%).
[0167] Step 2:
[0168]
[0169] Reference is ma...
Embodiment 2
[0173] Example 2: 3-((2S,5R)-4-oxo-5-(2-oxo-2-((1,1,2,2-d 4 -2-(3,4-dimethoxyphenyl)ethyl)amino)ethyl)-2-(4-(phenylethynyl)phenyl)thiazolidin-3-yl)benzamide
[0174]
[0175] To 3-((2S,5R)-4-oxo-5-(2-oxo-2-((1,1,2,2- d 4 -2-(4-hydroxy-3-methoxyphenyl)ethyl)amino)ethyl)-2-(4-(phenylethynyl)phenyl)thiazolidin-3-yl)benzamide ( Example 1) (25.00 mg; 0.04 mmol; 1.00 eq.) Potassium carbonate (0.04 g; 0.27 mmol; 6.50 eq.) and dimethyl sulfate (0.18 μl; 0.008 mmol; 0.04 eq.) were added. The reaction was stirred overnight at room temperature. The desired product was isolated by flash chromatography (10 g, KPNH, 0 to 20% methanol / DCM) to give the product as a white solid (8.9 mg, 35%).
[0176] 1 H NMR (500MHz, cd 3 od) δ7.83–7.78 (m, 1H), 7.69–7.64 (m, 1H), 7.50–7.30 (m, 11H), 6.81 (dd, J=5.0, 3.1, 2H), 6.74 (dd, J= 8.2, 2.0, 1H), 6.42(s, 1H), 4.53–4.47(m, 1H), 3.76(t, J=4.6, 6H), 3.10(dd, J=15.3, 4.1, 1H), 2.89(dd , J=15.3, 8.5, 1H).
[0177] m / z: 624; 625 [M+H] + .
Embodiment 3
[0178] Example 3: 3-((2S,5R)-4-oxo-5-(2-oxo-2-((1,1,2,2-d 4 -2-(3-methoxy-4-(methoxy-d 3 )phenyl)ethyl)amino)ethyl)-2-(4-(phenylethynyl)phenyl)thiazolidin-3-yl)benzamide
[0179]
[0180] According to the procedure similar to Example 2, from 3-((2S,5R)-4-oxo-5-(2-oxo-2-((1,1,2,2-d 4 -2-(4-hydroxy-3-methoxyphenyl)ethyl)amino)ethyl)-2-(4-(phenylethynyl)phenyl)thiazolidin-3-yl)benzamide ( 35.00mg; 0.06mmol; 1.00eq.) and dimethyl sulfate-d 6 (2.92 μl; 0.03 mmol; 0.50 eq.) to prepare the product. The reaction was stirred overnight at room temperature. The desired product was isolated by flash chromatography (10 g, KPNH, 0 to 20% methanol / DCM) to give the product as a white solid (22.3 mg, 63%).
[0181] 1 H NMR (500MHz, cd 3 od) δ7.83–7.79 (m, 1H), 7.70–7.65 (m, 1H), 7.48–7.30 (m, 11H), 6.83–6.77 (m, 2H), 6.74 (dd, J=8.2, 2.0, 1H), 6.42(s, 1H), 4.53–4.47(m, 1H), 3.75(s, 3H), 3.10(dd, J=15.2, 4.1, 1H), 2.89(dd, J=15.3, 8.5, 1H ).
[0182] m / z: 626; 627 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com