Compositions and methods for silencing aldehyde dehydrogenase
一种醛脱氢酶、组合物的技术,应用在用于沉默醛脱氢酶的组合物和方法领域,能够解决低病人依从性、双硫仑限制、不常见等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0321] VI. Preparation of Lipid Particles
[0322] Lipid particles of the invention, e.g., SNALPs, wherein nucleic acids such as interfering RNA (e.g., siRNA) are embedded within the lipid portion of the particle and protected from degradation, can be formed by any method known in the art, the Methods include, but are not limited to, continuous mixing, direct dilution, and in-line dilution.
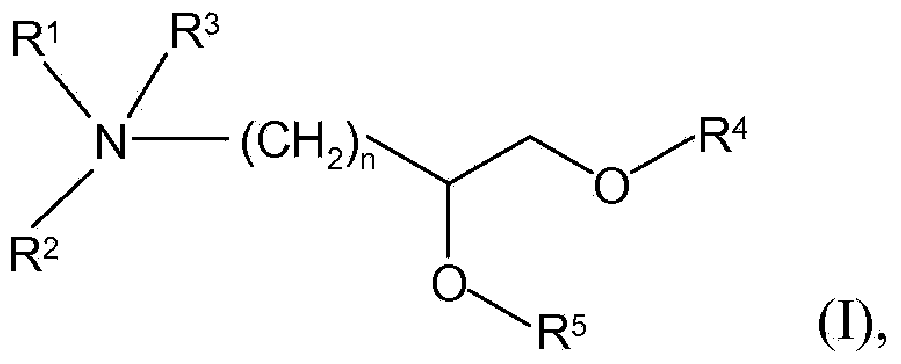
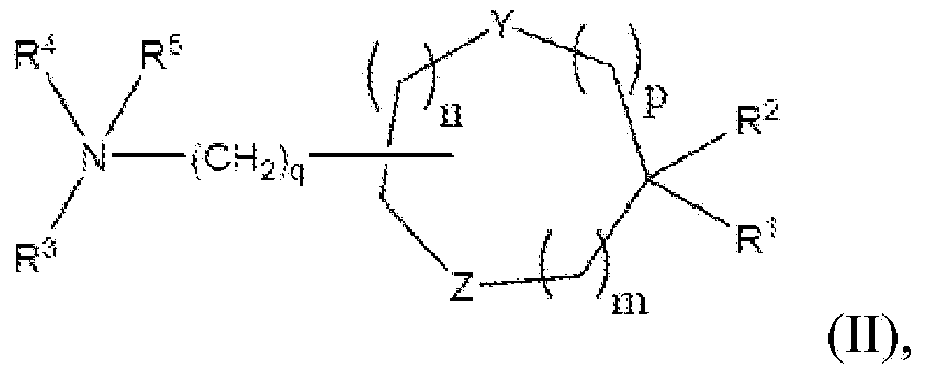
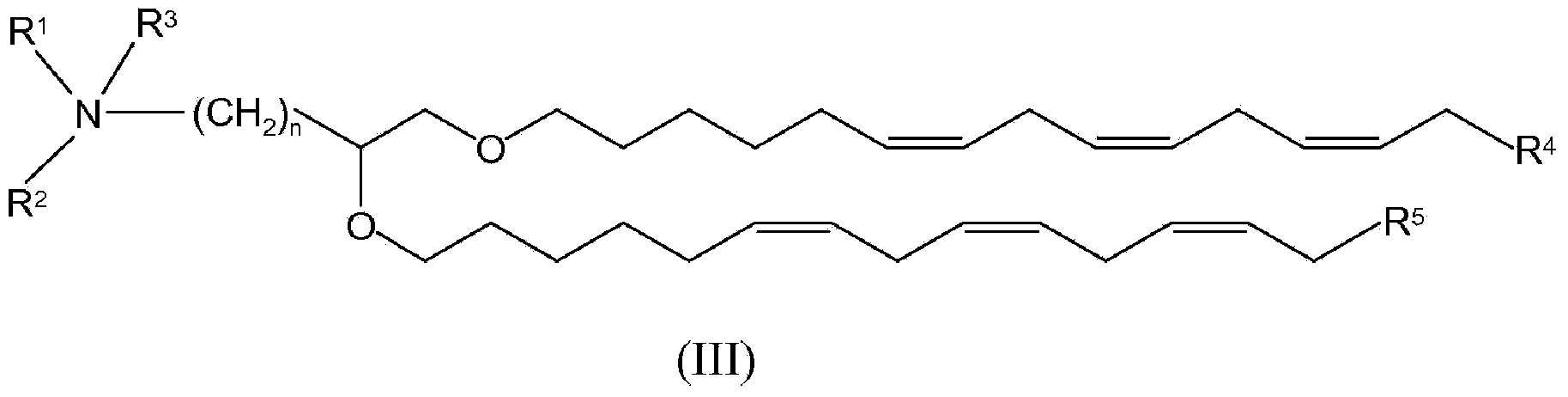
[0323] In specific embodiments, the cationic lipid may comprise a lipid of Formulas I-III or a salt thereof, alone, or in combination with other cationic lipids. In other embodiments, the non-cationic lipid is egg yolk sphingomyelin (ESM), distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylcholine (DOPC), 1-palmitoyl-2-oleoyl - Phosphatidylcholine (POPC), dipalmitoyl-phosphatidylcholine (DPPC), monomethyl-phosphatidylethanolamine, dimethyl-phosphatidylethanolamine, 14:0PE (1,2-dimyristoyl- Phosphatidylethanolamine (DMPE)), 16:0PE (1,2-dipalmitoyl-phosphatidylethanolamine (DPPE)),...
Embodiment 1
[0387] This example demonstrates that serum stable nucleic acid-lipid particles (SNALPs) containing siRNA targeting ALDH2 reduce aldehyde dehydrogenase 2 gene expression in murine hepatic cell lines in vitro.
[0388] Material:
[0389] All siRNA molecules used in these studies were chemically synthesized and annealed using standard procedures.
[0390] siRNA sequences targeting aldehyde dehydrogenase 2 (ALDH2) used in this study:
[0391]
[0392] where 'r' denotes ribonucleotides, 'm' denotes 2'-O-methylated ribonucleotides, and without any prefix denotes deoxynucleotides.
[0393] Additionally, non-targeting siRNA was included in the study as a control. This siRNA targets the firefly luciferase gene and is not intended to have any specific gene silencing activity in mammalian cells.
[0394] method:
[0395] Nucleic acid-lipid particles consisted of the following "1:57" formulation: 1.4% PEG2000-C-DMA; 57.1% DLinDMA; 7.1% DPPC; and 34.3% cholesterol. Typically, in a...
Embodiment 2
[0405] This example shows that SNALP comprising siRNA targeting ALDH2 reduces aldehyde dehydrogenase 2 gene expression in a whole animal system by the intravenous route of administration.
[0406] Material:
[0407] The mouse ALDH-2 siRNA duplex 1 and non-targeting control siRNA used in this study are described in Example 1.
[0408] method:
[0409] SNALP formulations were prepared as described in Example 1.
[0410] Animal Handling:
[0411] Female BALB / c mice were obtained from Harlan Labs. Animals were administered a single dose of SNALP formulated siRNA, or phosphate-buffered saline by intravenous injection of 10 mL / kg in the lateral tail vein. The siRNA dose administered was 0.025, 0.050 or 0.25 mg / kg body weight. Approximately 48 h after SNALP injection, animals were euthanized and liver tissues were collected in RNA stabilizing solution.
[0412] Organizational Analysis:
[0413]Essentially as described in Judge et al., 2006, Molecular Therapy (Molecular Thera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com