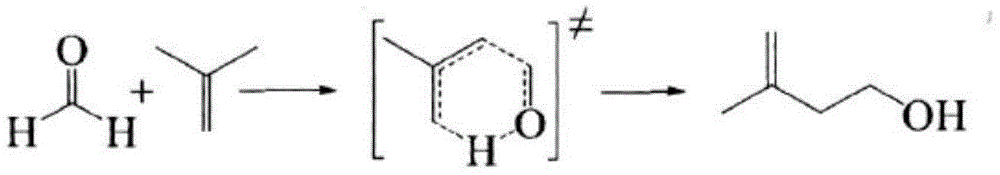
Preparation method for synthesizing 3-methyl-3-butene-1-ol by using formaldehyde hemiacetal
A formaldehyde hemiacetal, methyl technology, applied in the preparation of organic compounds, the preparation of hydroxyl compounds, chemical instruments and methods, etc., can solve problems such as low conversion rate of formaldehyde, achieve simple reaction raw materials, less by-products, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
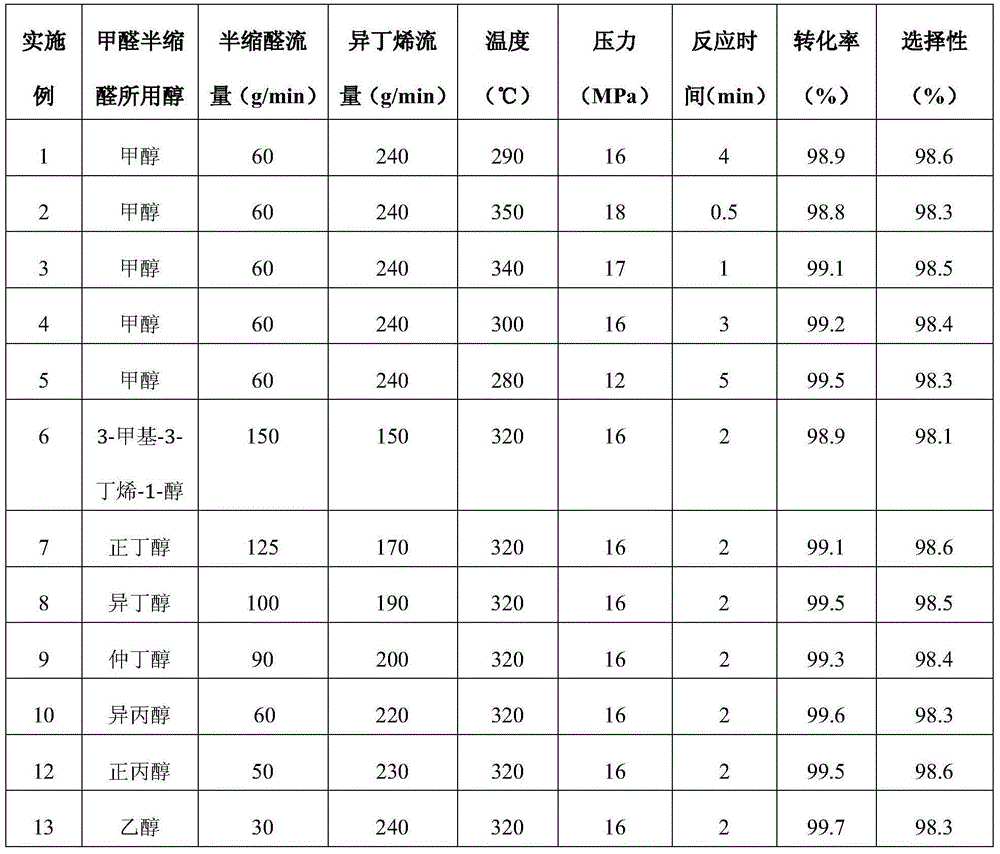
[0047] Under continuous operating conditions, pressurize 60g / min formaldehyde methanol hemiacetal to 16MPa and preheat to 290°C to reach the supercritical state of formaldehyde methanol hemiacetal. (240g / min) rapid mixing, supercritical reaction was carried out in the tubular reactor for 4 minutes, the reaction solution was decompressed and cooled, and after recovering isobutene, 3-methyl-3-butene-1 was detected by gas chromatography internal standard method -alcohol content, the conversion rate of formaldehyde methanol hemiacetal is 98.9%, and the selectivity of 3-methyl-3-buten-1-ol for formaldehyde methanol hemiacetal is 98.6%.
Embodiment 2-13
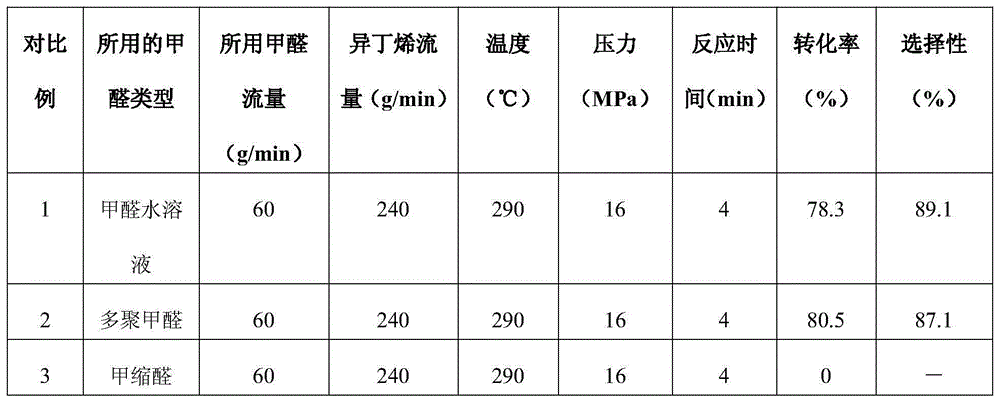
[0049] According to the operation method of embodiment 1, conditions such as the ratio of changing reaction temperature, reaction pressure, reaction time, isobutylene and formaldehyde hemiacetal respectively, the reaction solution obtained, its detection data are as shown in table 1, wherein conversion rate and 3- Methyl-3-buten-1-ol selectivities are all calculated for formaldehyde hemiacetal.
[0050] Table 1
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com