Indolone derivatives having different positions substituted and application thereof
A kind of derivative, indolinone technology, applied in the new compound preparation method and application field, can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] In order to understand the present invention, the following examples will further illustrate the present invention; the following examples are illustrative and not restrictive, and the scope of protection of the present invention cannot be limited by the following examples.
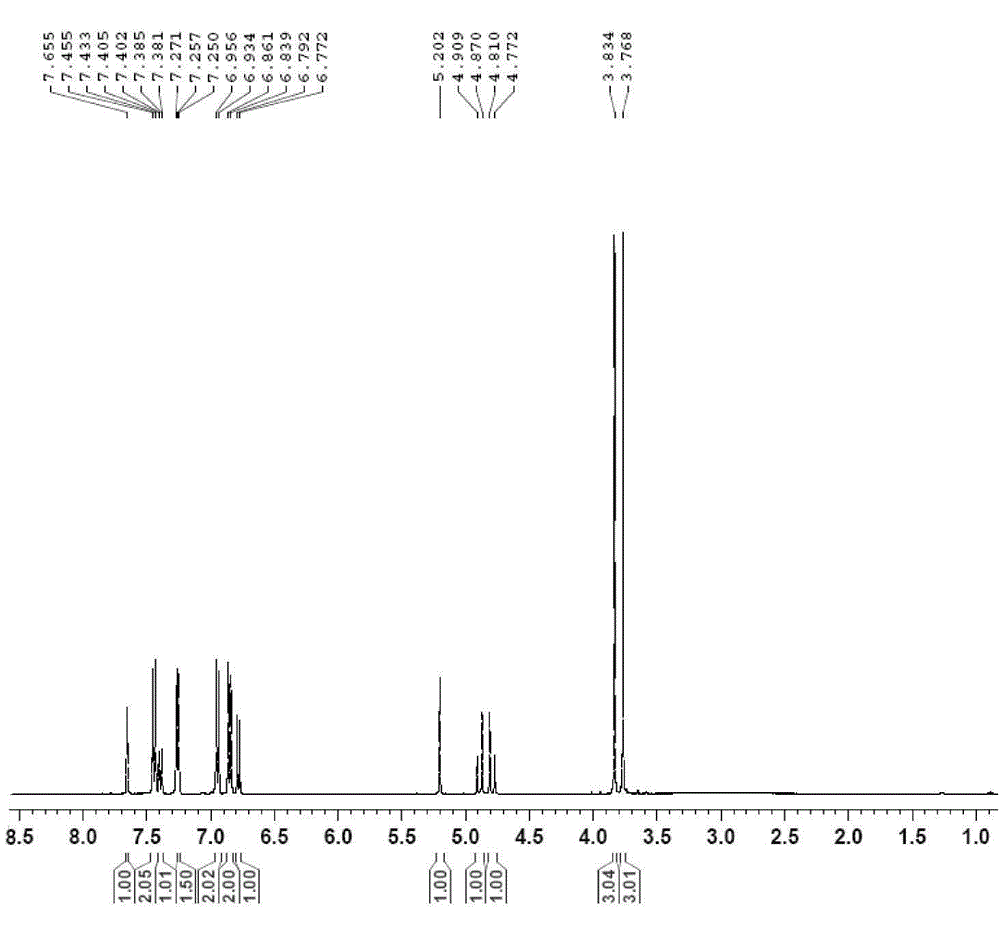
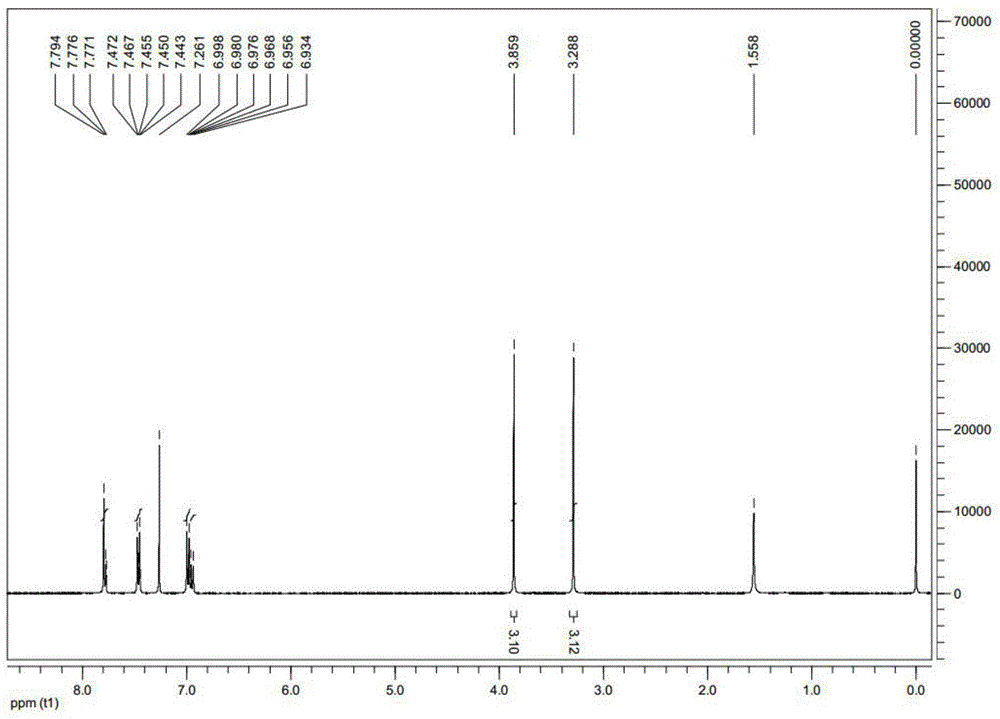
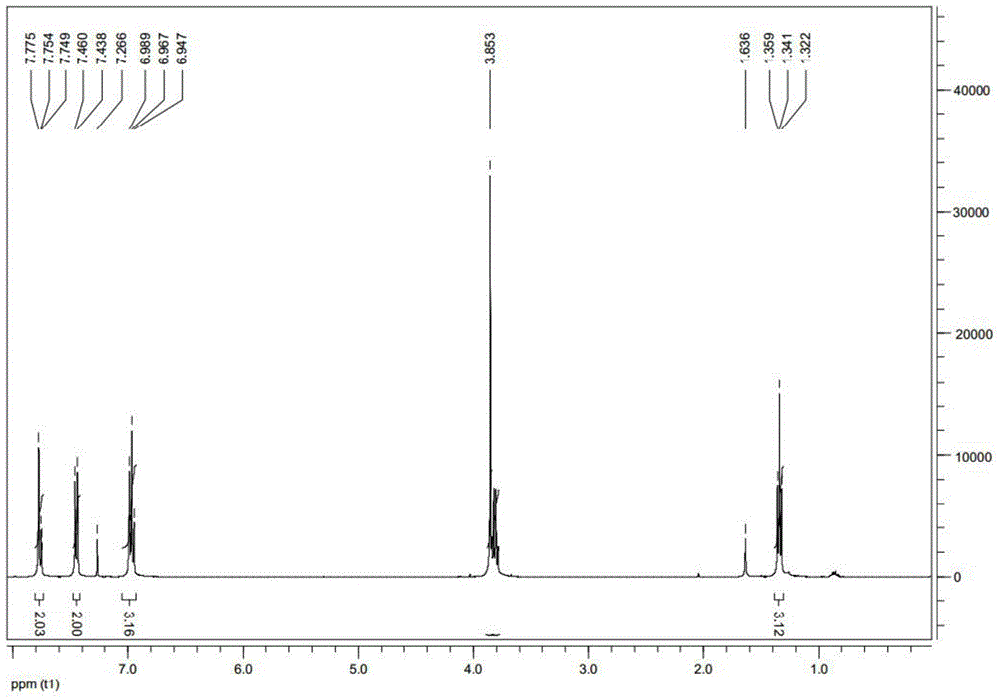
[0025] The anti-tumor drug of the compound of formula (I) of the present invention is designed to use indole dione as the parent ring, and the biological activity of the compound is improved by modifying the 1,5 position, and introducing halogen, alkyl, and aromatic ring at the 5 position. , Five-membered heterocycle, six-membered heterocycle to study the influence of space size, electronic effect and chain length on activity, introduce alkyl and different substituted benzyl groups at the 1 position to study the influence factors of the N-1 group ; Then, the above-mentioned modification was performed on the 1,4, 1,6, and 1,7 positions to study the effect of different positions on the anti-tumor activit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com