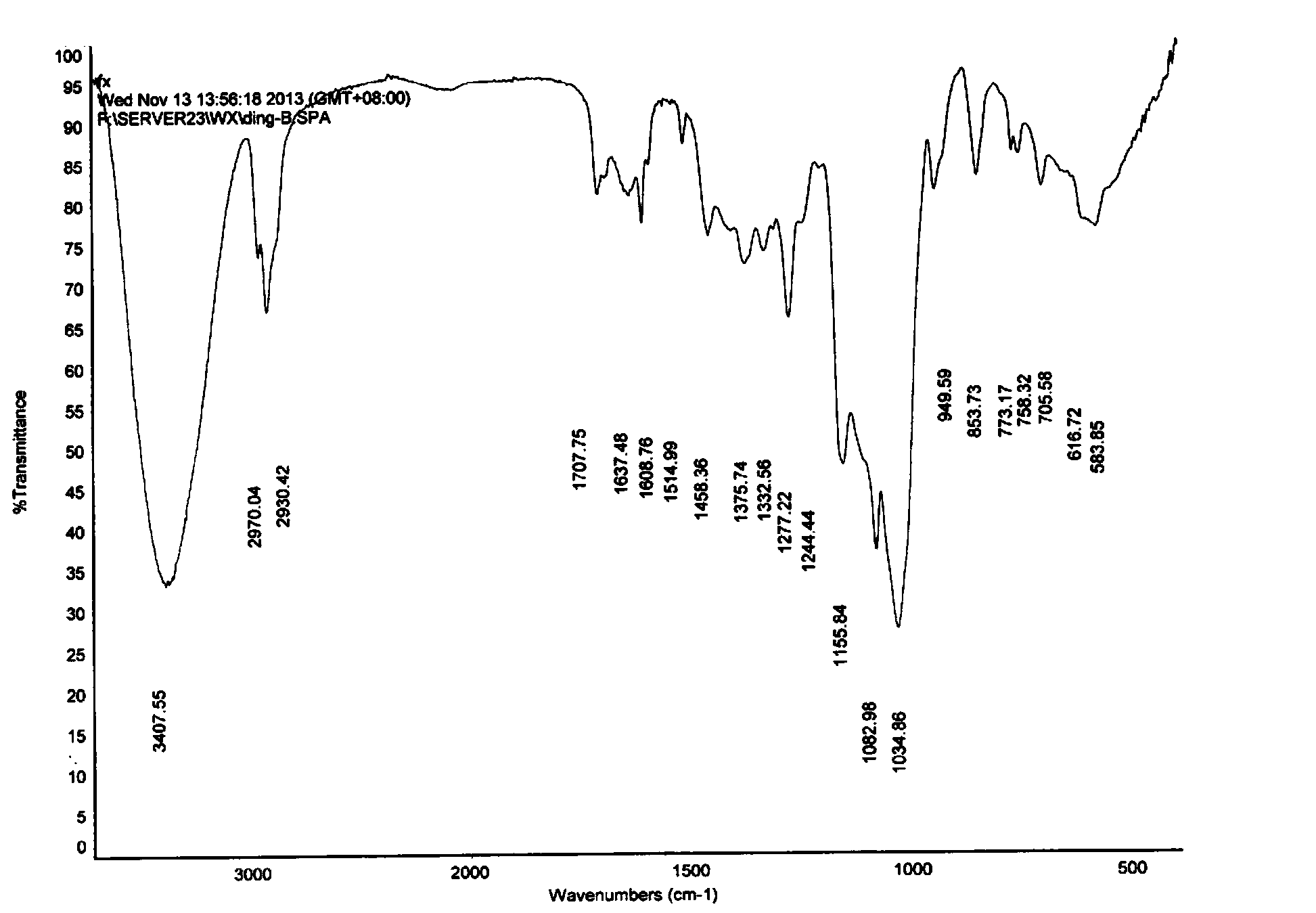
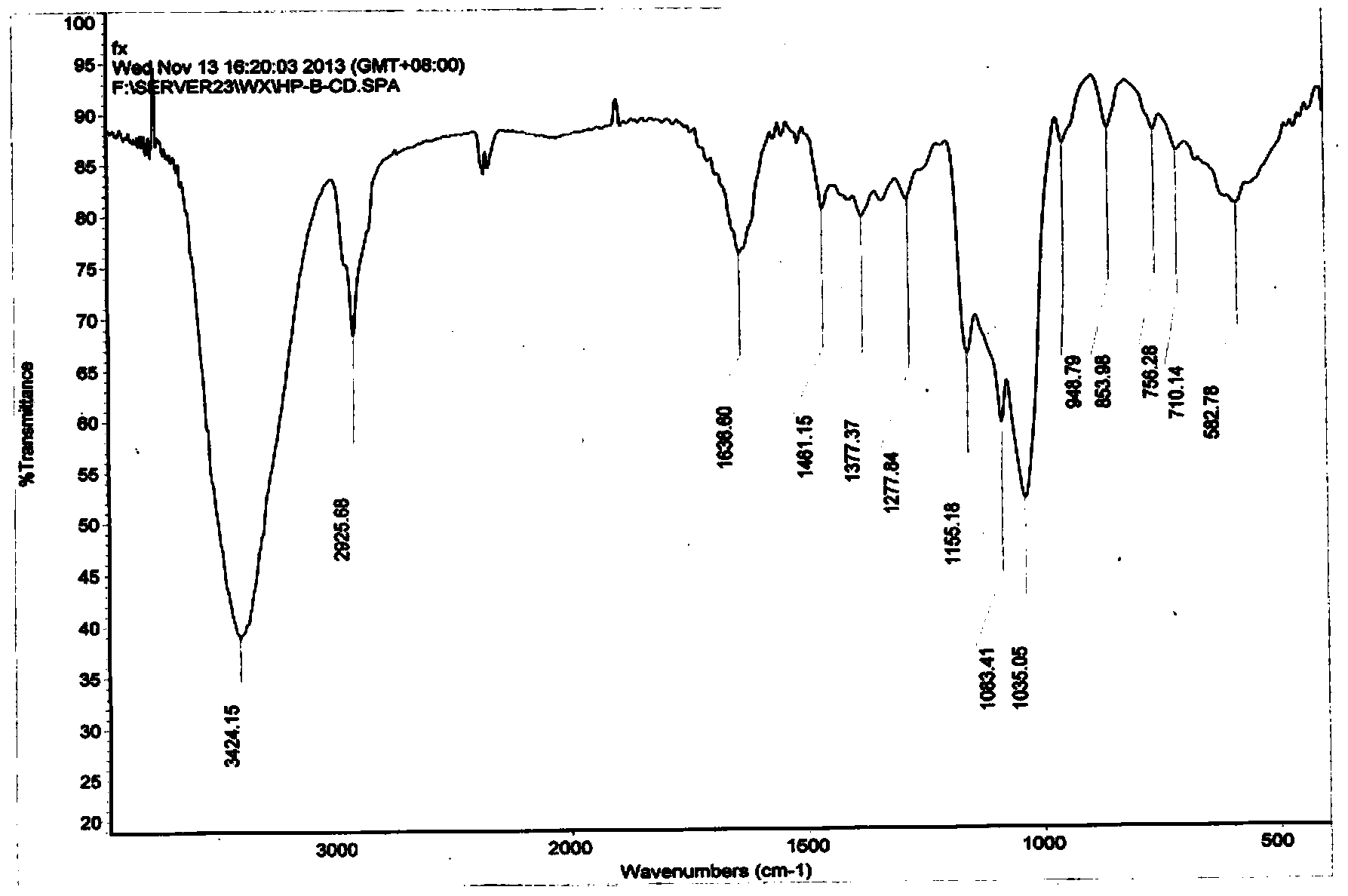
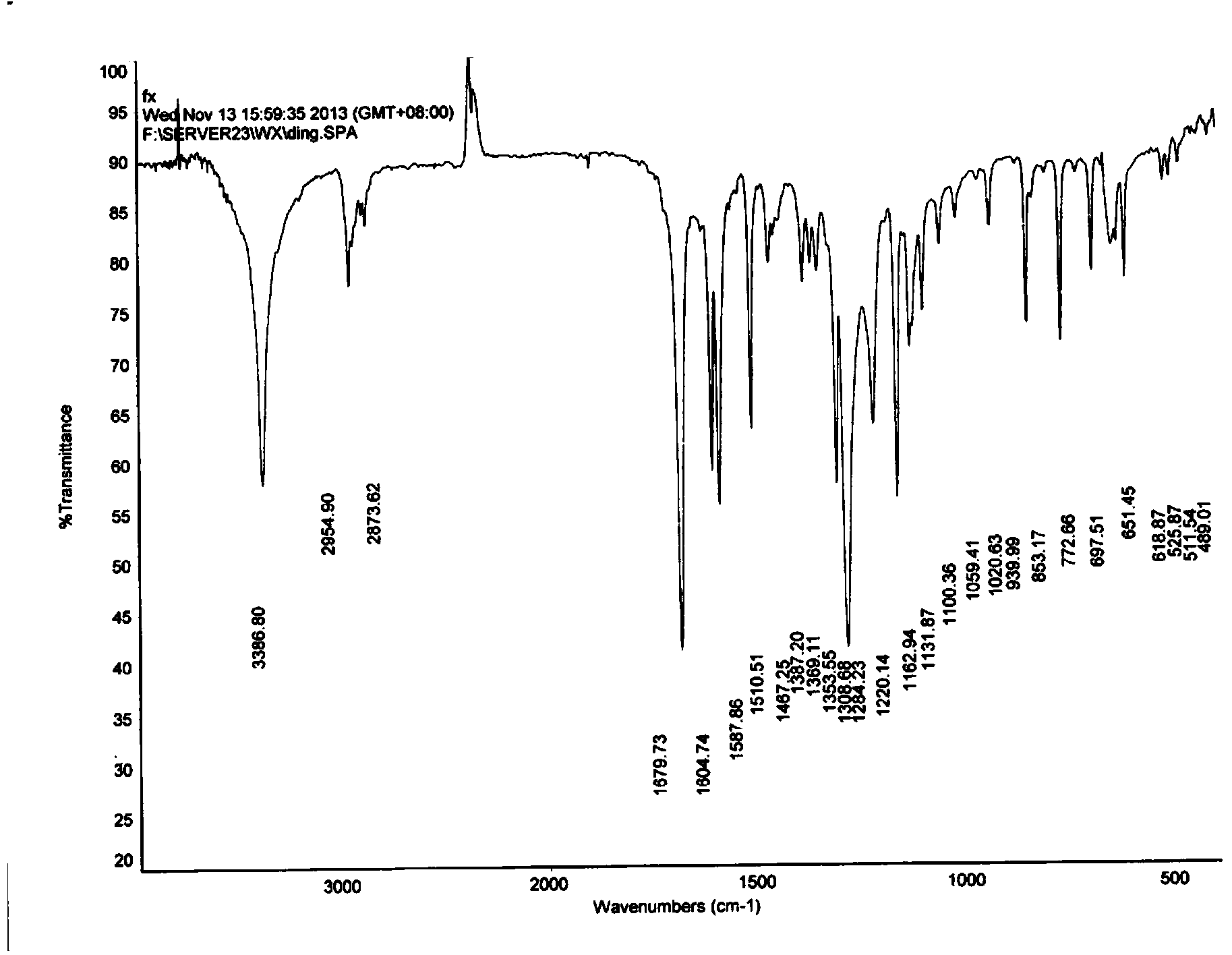
Hydroxypropyl-beta-cyclodextrin supermolecule clathrate compound of butyl p-hydroxybenzoate, and preparing method and using method thereof
A technology of butyl hydroxybenzoate and hydroxypropyl, which is applied in the field of supramolecular inclusion compound of hydroxypropyl-β-cyclodextrin, can solve the problem of affecting the use of butyl hydroxybenzoate, antibacterial and antibacterial efficacy, and affecting convenience , safety, organic solvents are not easy to remove, etc., to solve the problems of difficult application and operation, low toxicity, antibacterial and antibacterial effects, large solubility and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The hydroxypropyl-β-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, by weight, butyl p-hydroxybenzoate:hydroxypropyl-β-cyclodextrin=1:10 .
[0031] In this example, the hydroxypropyl-β-cyclodextrin is 2-hydroxypropyl-β-cyclodextrin, and the hydroxypropyl derivatives of other β-cyclodextrins with similar physical and chemical properties (3-hydroxypropyl Base-β-cyclodextrin) instead of 2-hydroxypropyl-β-cyclodextrin has no significant effect on the performance of the product.
[0032] The preparation method of the hydroxypropyl-beta-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, the hydroxypropyl-beta-cyclodextrin is mixed with the hydroxypropyl-beta-cyclodextrin by weight ratio Dextrin: water=0.5 is dissolved in the water that temperature is 80 ℃ and adds the butyl p-hydroxybenzoate of setting weight ratio, then constantly stirs under heating or insulation until butyl p-hydroxyben...
Embodiment 2
[0036] The hydroxypropyl-β-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, by weight, butyl p-hydroxybenzoate:hydroxypropyl-β-cyclodextrin=1:20 .
[0037] In this example, the hydroxypropyl-β-cyclodextrin is 3-hydroxypropyl-β-cyclodextrin, and the hydroxypropyl derivatives of other β-cyclodextrins with similar physical and chemical properties (2-hydroxypropyl 3-hydroxypropyl-β-cyclodextrin) instead of 3-hydroxypropyl-β-cyclodextrin has no obvious effect on the performance of the product.
[0038] The preparation method of the hydroxypropyl-beta-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, the hydroxypropyl-beta-cyclodextrin is mixed with the hydroxypropyl-beta-cyclodextrin by weight ratio Dextrin: water=0.3 is dissolved in the water of 70 ℃ and adds the butyl p-hydroxybenzoate of setting weight ratio after being dissolved in water, then constantly stirs under heating or insulation until...
Embodiment 3
[0042] The hydroxypropyl-β-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, by weight, butyl p-hydroxybenzoate:hydroxypropyl-β-cyclodextrin=1:30 .
[0043] In this example, the hydroxypropyl-β-cyclodextrin is 3-hydroxypropyl-β-cyclodextrin, and the hydroxypropyl derivatives of other β-cyclodextrins with similar physical and chemical properties (2-hydroxypropyl 3-hydroxypropyl-β-cyclodextrin) instead of 3-hydroxypropyl-β-cyclodextrin has no obvious effect on the performance of the product.
[0044] The preparation method of the hydroxypropyl-beta-cyclodextrin supramolecular inclusion complex of butyl p-hydroxybenzoate in this embodiment, the hydroxypropyl-beta-cyclodextrin is mixed with the hydroxypropyl-beta-cyclodextrin by weight ratio Dextrin: water=1, be dissolved in the water that temperature is 65 ℃ and add the butyl p-hydroxybenzoate of setting weight ratio, then constantly stir under heating or insulation until butyl p-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com