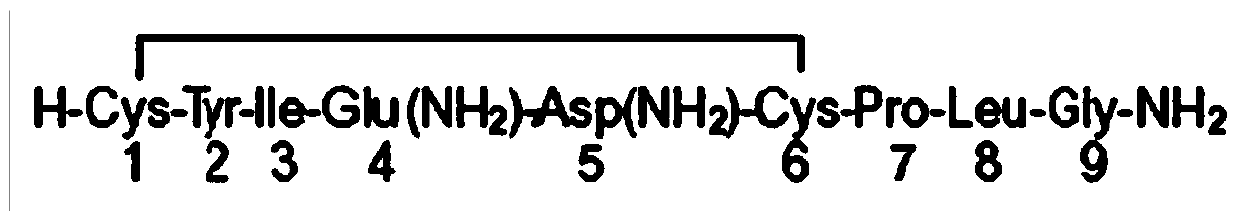Compound drug composition for delivery and application of compound drug composition
A composition and drug technology, applied in the field of medicine, can solve the problems of short plasma half-life of oxytocin, short duration of uterine contraction effect, rise in body blood pressure, etc., and achieve small adverse reactions, strong activity, optimal onset time and duration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] experiment material
[0032] Test drug:
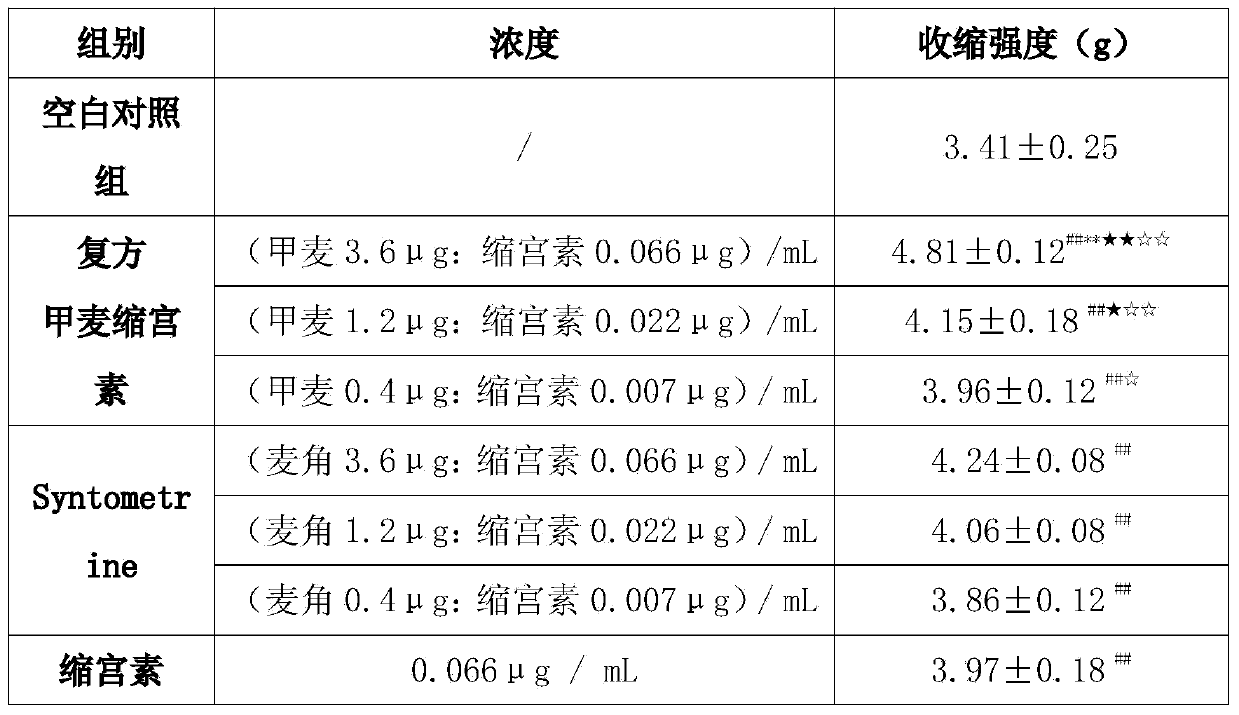
[0033] Oxytocin, white powder, content: 99.7%, batch number: 1311035, provided by Nanjing Jiqun Pharmaceutical Technology Co., Ltd., 1mg=547IU. Before use, weigh 36IU / 66μg, 12IU / 22μg, 4IU / 7μg oxytocin powder and dissolve them in 10ml normal saline respectively to obtain 3.6IU / ml, 1.2IU / ml, 0.4IU / ml oxytocin injection. Oxytocin injection needs to be prepared and used immediately, and the sample should be stored in the dark at 2-8°C.
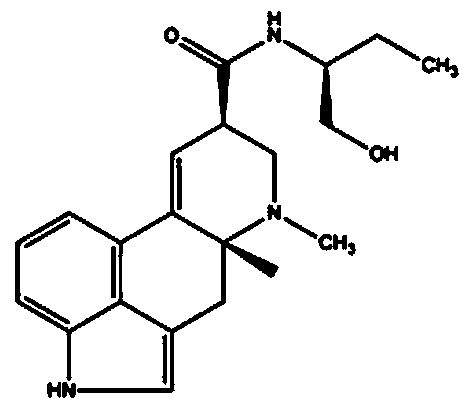
[0034] Methergonovine maleate, content: 100.1%, batch number: 72621000213, provided by Nanjing Jiqun Pharmaceutical Technology Co., Ltd. Before use, weigh 3.6mg, 1.2mg, 0.4mg methysergide maleate powder and dissolve them in 10ml normal saline respectively to obtain 360μg / ml, 120μg / ml, 40μg / ml methysergide maleate injection . Methergometrine injection needs to be prepared and used immediately, and the sample should be stored in the dark at 2-8°C.
[0035]Compound methylergide injection, drug pr...
Embodiment 2
[0114] Example of prescription and preparation process of compound methylergometrine oxytocin injection:
[0115] The drug specifications are: methylergonovine maleate 0.5mg / mL, oxytocin 5I.U. / mL, 1mL / branch.
[0116]
[0117] Get 0.5 g of methylergonovine maleate, 5000 I.U. of oxytocin and 7 g of sodium chloride, add them to 800 ml of water for injection, stir until all are dissolved, and adjust the pH to 2.5 to 5. After adding activated carbon for adsorption, add water to make up to 1000ml, filter and sterilize with microporous membrane method, then quantitatively fill 1ml into an ampoule bottle to obtain the product. According to clinical needs, choose intramuscular or intravenous injection.
Embodiment 3
[0119] Compound methylergonovine maleate oxytocin injection prescription and preparation process example:
[0120] The drug specifications are: methylergonovine maleate 1mg / mL, oxytocin 5I.U. / mL, 1mL / branch.
[0121]
[0122] Take 1g of methylergonovine maleate, 5000I.U. of oxytocin and 7g of sodium chloride, add them to 800ml of water for injection, stir until they are completely dissolved, and adjust the pH to 2.5 to 5. After adding activated carbon for adsorption, add water to make up to 1000ml, filter and sterilize with microporous membrane method, then quantitatively fill 1ml into an ampoule bottle to obtain the product. According to clinical needs, choose intramuscular or intravenous injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com