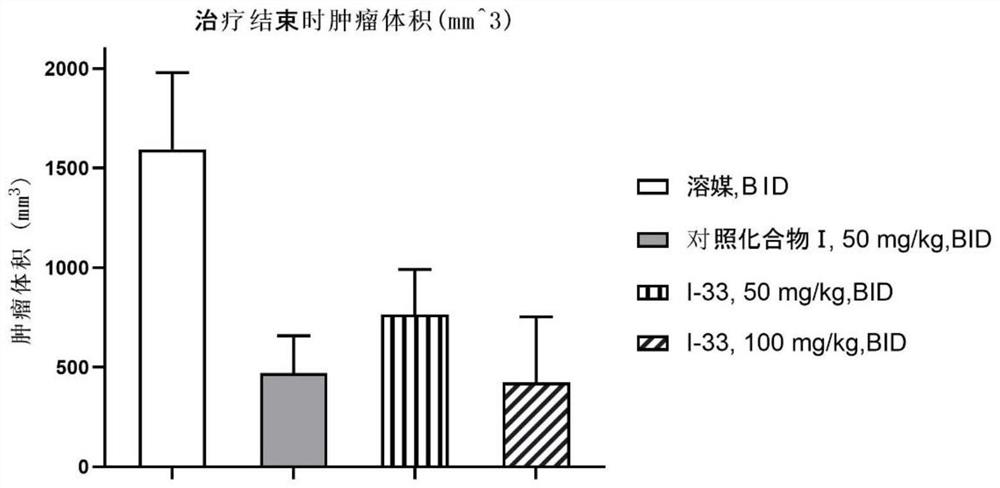
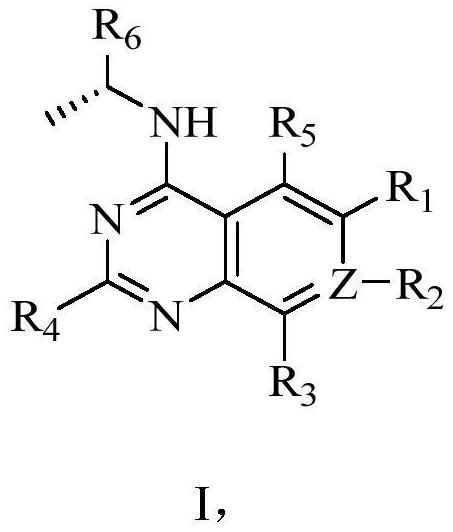
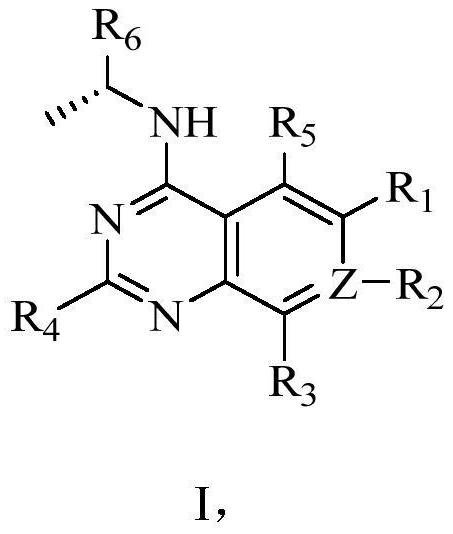
6-substituted phosphoryl quinazoline derivative as well as preparation method and application thereof
A phosphoryl quinazoline and derivative technology, applied in chemical instruments and methods, active ingredients of phosphorus compounds, drug combinations, etc., can solve problems such as differences in biological functions, and achieve low toxicity, high safety, and druggability. Effects with low risk of drug-drug interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0343] Example 1: Preparation of Compound I-1
[0344] The synthetic route is as follows:
[0345]
[0346] The first step: the synthesis of methyl 2-amino-4-methoxybenzoate (B1-2)
[0347]
[0348] To a solution of compound B1-1 (5 g, 29.91 mmol) in methanol (60 mL) was added dropwise thionyl chloride (299 mmol, 21.7 mL) at 5°C, and the reaction solution was reacted at 70°C for 12 hours. The reaction solution was concentrated under reduced pressure to obtain a crude product, which was purified by beating with ethyl acetate, and filtered to obtain compound B1-2 (4.34 g, yield 80%).
[0349] The second step: the synthesis of methyl 2-amino-5-iodo-4-methoxybenzoate (B1-3)
[0350]
[0351] To a solution of Compound B1-2 (4 g, 22.1 mmol) in DMF (60 mL) was added N-iodosuccinimide (5.22 g, 23.2 mmol) at room temperature, followed by reaction at room temperature for 12 hours. The reaction solution was concentrated under reduced pressure to obtain a crude product, which wa...
Embodiment 2
[0363] Example 2: Preparation of Compound 1-2
[0364] (R)-Diethyl(7-methoxy-2-methyl-4-((1-(2-methyl-3-(trifluoromethyl)phenyl)ethyl)amino)quinazoline Synthesis of -6-yl)phosphine oxide (I-2)
[0365]
[0366] Compound B1-5 (100 mg, 0.2 mmol) was dissolved in DMF (3 mL), and diethylphosphine oxide (31 mg, 0.29 mmol), tris(dibenzylideneacetone)dipalladium (0.016 mmol, 14.7 mmol) were added under nitrogen protection mg), 4,5-bis(diphenylphosphonium)-9,9-dimethylxanthene (0.032 mmol, 18.5 mg) and triethylamine (0.08 mL, 0.6 mmol). The reaction solution was reacted at 90°C. After the reaction was completed, the reaction solution was directly concentrated to obtain the crude product. The crude product was separated and purified by column chromatography to obtain the target compound I-2 (75 mg, yield 78%).
[0367] 1 H NMR (400MHz, CDCl 3 ):δ8.38(d,1H),7.62-7.52(m,1H),7.28-7.24(m,1H),7.10(d,1H),6.34(d,1H),5.87-5.80(m,1H) ),3.92(s,3H),2.63(d,3H),2.52(s,3H),2.14-1.96(m,4H),...
Embodiment 3
[0369] Example 3: Preparation of Compound 1-3
[0370] The synthetic route is as follows:
[0371]
[0372] The first step: (R)-N-(1-(3-(difluoromethyl)-2-fluorophenyl)ethyl)-6-iodo-7-methoxy-2-methylquinazoline Synthesis of -4-amine (B3-1)
[0373]
[0374] To a solution of compound B1-4 (350 mg, 1.11 mmol) in dichloromethane (9 mL) was added 2,4,6-triisopropylbenzenesulfonyl chloride (368 mg, 1.22 mmol), 4-dimethylaminopyridine (13.4 mg) , 0.11 mmol), triethylamine (0.46 mL, 3.33 mmol). The reaction solution was then reacted at room temperature overnight. The reaction solution was concentrated under reduced pressure to obtain the crude product, and triethylamine (0.46 mL, 3.33 mmol) and (R)-1-(3-(difluoromethyl)-2-fluorophenyl)ethane were added to the DMSO solution of the crude product -1-amine hydrochloride (250 mg, 1.11 mmol), then reacted at 80°C overnight. The reaction solution was cooled to room temperature. Water was added to the reaction solution, extracte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com