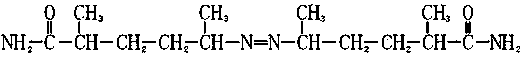Preparation method for dinitrogen heptyl amide
A technology of bisazeptanamide and aminoheptanenitrile, applied in directions such as organic chemistry, can solve problems such as the large influence of catalyst decomposition rate, and achieve the effects of low production cost, high product purity, and small oxidizing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The preparation method of diazepamamide of the present invention, its steps are as follows:
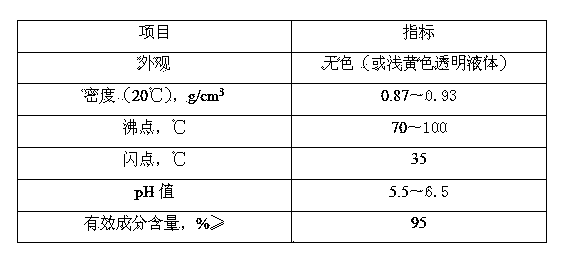
[0014] A. Raw materials and proportions include: 10 parts of aminoheptanonitrile, 50 parts of 15% sodium hydroxide solution, 2 parts of chlorine gas, 40 parts of ethanol, 10 parts of N,N-dimethylformamide, and 0.5 parts of sodium methoxide and 5 parts of liquefied ammonia, all of which are parts by weight;
[0015] B. Mix 10 parts of aminoheptanitril with 50 parts of 15% sodium hydroxide solution, feed 2 parts of chlorine gas at 0°C within 1 hour, cool down to -5°C, and let it stand at constant temperature for 8 hours. solid sediment;
[0016] C. The solid precipitate obtained in step B is heated and dried at 20°C for 8 hours, and when its water content is ≤3%, add it to 40 parts of ethanol, then add 10 parts of N,N-dimethylformamide and 0.5 Parts of catalyst sodium methoxide, 5 parts of liquid ammonia were added within 5 hours, and after standing at 15°C for 12 hours, the te...
Embodiment 2
[0018] The preparation method of diazepamamide of the present invention, its steps are as follows:
[0019] A. Raw materials and proportions include: 40 parts of aminoheptanitril, 120 parts of 15% sodium hydroxide solution, 8 parts of chlorine gas, 100 parts of ethanol, 40 parts of N,N-dimethylformamide, and 1 part of sodium methoxide And 20 parts of liquefied ammonia, all above are parts by weight;
[0020] B. Mix 40 parts of aminoheptanitril with 120 parts of 15% sodium hydroxide solution, feed 8 parts of chlorine gas at 10°C within 8 hours, cool down to -20°C, let stand at constant temperature for 15 hours, and take whichever solid sediment;
[0021] C. Dry the solid precipitate obtained in step B at 50°C for 15 hours. When its water content is ≤3%, add it to 100 parts of ethanol, then add 40 parts of N,N-dimethylformamide and 1 Parts of catalyst sodium methoxide, add 20 parts of liquid ammonia within 10 hours, stand at 20°C for 36 hours, lower the temperature to -50°C to...
Embodiment 3
[0023] The preparation method of diazepamamide of the present invention, its steps are as follows:
[0024] A. Raw materials and proportions include: 20 parts of aminoheptanonitrile, 80 parts of 15% sodium hydroxide solution, 4 parts of chlorine gas, 50 parts of ethanol, 15 parts of N,N-dimethylformamide, and 0.6 parts of sodium methoxide and 8 parts of liquefied ammonia, all of which are parts by weight;
[0025] B. Mix 20 parts of aminoheptanonitrile with 80 parts of 15% sodium hydroxide solution, feed 4 parts of chlorine gas at 5°C within 5 hours, lower the temperature to -5°C, and let it stand at constant temperature for 10 hours. solid sediment;
[0026] C. The solid precipitate obtained in step B is heated and dried at 30°C for 10 hours, and when its water content is ≤3%, add it to 50 parts of ethanol, then add 15 parts of N,N-dimethylformamide and 0.6 One part of catalyst sodium methoxide, 8 parts of liquid ammonia were added within 5 hours, and after standing at 15°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com