Method and model building method for predicting bovine serum albumin-water partition coefficient of organic compounds based on molecular structure
A bovine serum albumin and organic compound technology, which is applied in the fields of electrical digital data processing, special data processing applications, instruments, etc., can solve the problems of inability to use environmental organic compounds to predict, limited types of compounds, and low prediction reliability, and achieves practical results. The application ability is strong, the method is convenient and fast, and the effect is easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
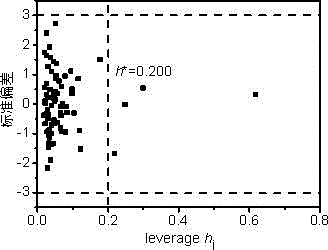
[0030] n-octane: calculated by Williams graph method h i Value is 0.065h *(warning value)=0.200, standard residual ( SE ) = -0.284 > -3, indicating that this compound is within the application domain of the QSAR model. Using the PM6 algorithm of MOPAC 2012, ChemOffice 2010 and EPI Suite calculated 5 descriptors respectively.
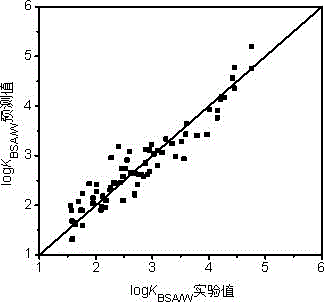
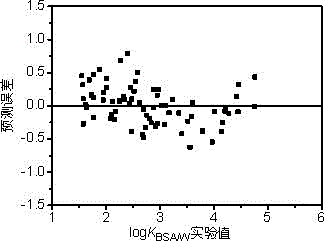
[0031] log of n-octane K BSA / W The experimentally determined value was 4.01 [L / kg]. The prediction steps based on the QSAR model are as follows:
[0032] log K BSA / W = 0.747×log K ow + 0.476× E HOMO - 0.024× CCR + 0.870× q + + 0.007× CSEV + 4.342
[0033] =0.747×(5.81)+0.476×(-10.434)-0.024×(19.173)+0.870×(0.078)+
[0034] 0.007×(145.454) + 4.342
[0035] =3.87.
Embodiment 2
[0037] Tetrachlorethylene: calculated by Williams graph method h i Value is 0.051h *(warning value)=0.200, standard residual ( SE ) = 2.732 < 3, indicating that this compound is within the application domain of the QSAR model. Using the PM6 algorithm of MOPAC 2012, ChemOffice 2010 and EPI Suite calculated 5 descriptors respectively.
[0038] log of tetrachlorethylene K BSA / W The experimentally determined value was 2.40 [L / kg]. The prediction steps based on the QSAR model are as follows:
[0039] log K BSA / W = 0.747×log K ow + 0.476× E HOMO - 0.024× CCR + 0.870× q + + 0.007× CSEV + 4.342
[0040] =0.747×(3.40)+0.476×(-9.545)-0.024×(-6.035)+0.870×(0.029)+
[0041] 0.007×(90.284) + 4.342
[0042] =3.14.
Embodiment 3
[0044] Pyrene: calculated by Williams graph method h i Value is 0.248> h *(warning value)=0.200, standard residual ( SE) = -0.016 > -3, indicating that this compound is in the application domain of the QSAR model, and the model has good generalization ability. Using the PM6 algorithm of MOPAC 2012, ChemOffice 2010 and EPI Suite calculated 5 descriptors respectively.
[0045] Pyrene's log K BSA / W The experimentally determined value was 4.76 [L / kg]. The prediction steps based on the QSAR model are as follows:
[0046] log K BSA / W = 0.747×log K ow + 0.476× E HOMO - 0.024× CCR + 0.870× q + + 0.007× CSEV + 4.342
[0047] =0.747×(4.88)+0.476×(-8.397)-0.024×(48.446)+0.870×(0.854) +
[0048] 0.007×(161.662) + 4.342
[0049] =4.70.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com