A method for measuring acidity of copper-containing ferric sulfuric acid solution
A technology of sulfuric acid solution and measurement method, which is applied in chemical analysis by titration, material analysis by observing the influence on chemical indicators, and analysis by making materials undergo chemical reactions, etc., which can solve the problem of inaccurate determination of acidity and titration The end point is not easy to judge and other problems, to achieve the effect of easy judgment and accurate acidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: A method for measuring the acidity of copper-iron sulfuric acid solution, first calculate the acidity when the content of copper and iron ions in the solution is 0 g / L.
[0017] 1. Measurement method
[0018] Pipette 1ml of sulfuric acid solution containing copper and iron into a 250ml Erlenmeyer flask, add 75ml~100ml of deionized water, add 0.5g of potassium oxalate, shake well to fully dissolve, add 6~10 drops of bromocresol green-methyl red Mix indicator and titrate with sodium hydroxide standard solution. During the titration, when the sulfuric acid solution changes from dark red to dark green, add two drops in excess, which is the end point.
[0019] 2. Instruments and reagents used
[0020] Acid-base universal burette: 50ml; conical flask: 250ml; single-marked pipette: 1ml;
[0021] Sodium hydroxide: superior grade; Potassium oxalate: analytical grade; bromocresol green-methyl red mixed indicator.
[0022] 3. Solution preparation
[0023] (1) 0.1...
Embodiment 2
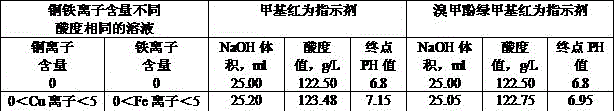
[0036] Embodiment 2: A method for measuring the acidity of a copper-iron sulfuric acid solution, when in the solution to be tested: 0g / L<copper ion content<5g / L, 0g / L<iron ion content<5g / L;
[0037] 1. Measurement method
[0038] Pipette 1ml of sulfuric acid solution containing copper and iron into a 250ml Erlenmeyer flask, add 75ml~100ml of deionized water, add 0.5g of potassium oxalate, shake well to fully dissolve, add 6~10 drops of bromocresol green-methyl red Mix the indicator and titrate with sodium hydroxide standard solution. When the sulfuric acid solution changes from dark red to dark green, add two drops in excess, which is the end point.
[0039] 2. Instruments and reagents used
[0040] Acid-base universal burette: 50ml; conical flask: 250ml; single-marked pipette: 1ml;
[0041] Sodium hydroxide: superior grade; Potassium oxalate: analytical grade; bromocresol green-methyl red mixed indicator.
[0042] 3. Solution preparation
[0043] (1) 0.1mol / L sodium hydro...
Embodiment 3
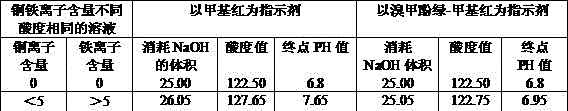
[0056] Embodiment three: the mensuration method of copper-containing iron sulfuric acid solution acidity, method is the same as embodiment two, but used test solution is different during mensuration: 0g / Lin test solution 5g / L,
[0057] Use methyl red indicator and bromocresol green-methyl red indicator respectively to measure the acidity of the solution; the solution with methyl red as indicator has turbidity, and the end color of the solution is yellow; The terminal color of the solution with phenol green-methyl red as indicator is bright green; the amount and acidity value of the sodium hydroxide standard solution consumed are as shown in Table 3, and the pH value of deionized water is 6.65.
[0058]
[0059]As can be seen from Table 3, when 0g / L5g / L in the solution, it can be seen from the volume, acidity value, and terminal pH value of the sodium hydroxide standard solution consumed : When methyl red is used as indicator, the acidity measurement result is larger than w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com