Fulvestrant-containing medicine preparation
A technology for fulvestrant and pharmaceutical preparations, applied in the field of pharmaceutical preparations, can solve the problems of increasing muscle stimulation at the injection site, the irritation of benzyl benzoate, slow adsorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Dissolution of fulvestrant in different solvents
[0041] Fulvestrant was dissolved in different solvents respectively, and the dissolution conditions are shown in Table 1:
[0042] Table 1 Dissolution of fulvestrant in different solvents (25°C)
[0043]
[0044]
[0045] From the above test, it can be known that the solubility of fulvestrant in castor oil is better than that in other oil bases, which is beneficial to the sustained release of the drug; fulvestrant has good solubility in NMP and ethanol, which are respectively 864mg / ml and >200mg / ml.
Embodiment 2
[0046] Example 2 Determination of sink conditions (37°C, determination of in vitro release method)
[0047] The solubilizing effect of different solubilizers on the drug in PBS7.4 was measured, so as to obtain the solubilizing agent that could meet the solubilizing effect of the sink condition.
[0048] Known from above-mentioned test: in vitro release condition is the PBS (pH7.4) damping fluid 500mL that contains 0.25% SDS, 37 ℃, 50rpm constant temperature water-bath shaker, 0.5mL (containing medicament 25mg) prescription, direct release method (sink condition: 5 times).
[0049] Table 2 Solubilization effect of solubilizer on FVT
[0050] Solubilizers
Embodiment 3
[0051] Example 3 Investigating the in vitro release of different formulations of fulvestrant
[0052] Prepare the prescription solution according to Table 3, and then filter the prepared solution with a 0.22 μm filter.
[0053] Table 3 Composition of different formulations of fulvestrant
[0054]
[0055] The above-mentioned several groups of preparation prescriptions were carried out in vitro release test according to the following conditions: in 500ml containing 0.25% sodium lauryl sulfate, pH7.4 PB buffer solution, add 0.5ml sample, under the condition of 37 ℃, at a constant temperature of 100rpm Keep warm with reciprocating vibration in a water bath shaker. Regular sampling and testing. The test results are as follows:
[0056] Table 4 In vitro release test results of different formulations of fulvestrant
[0057]
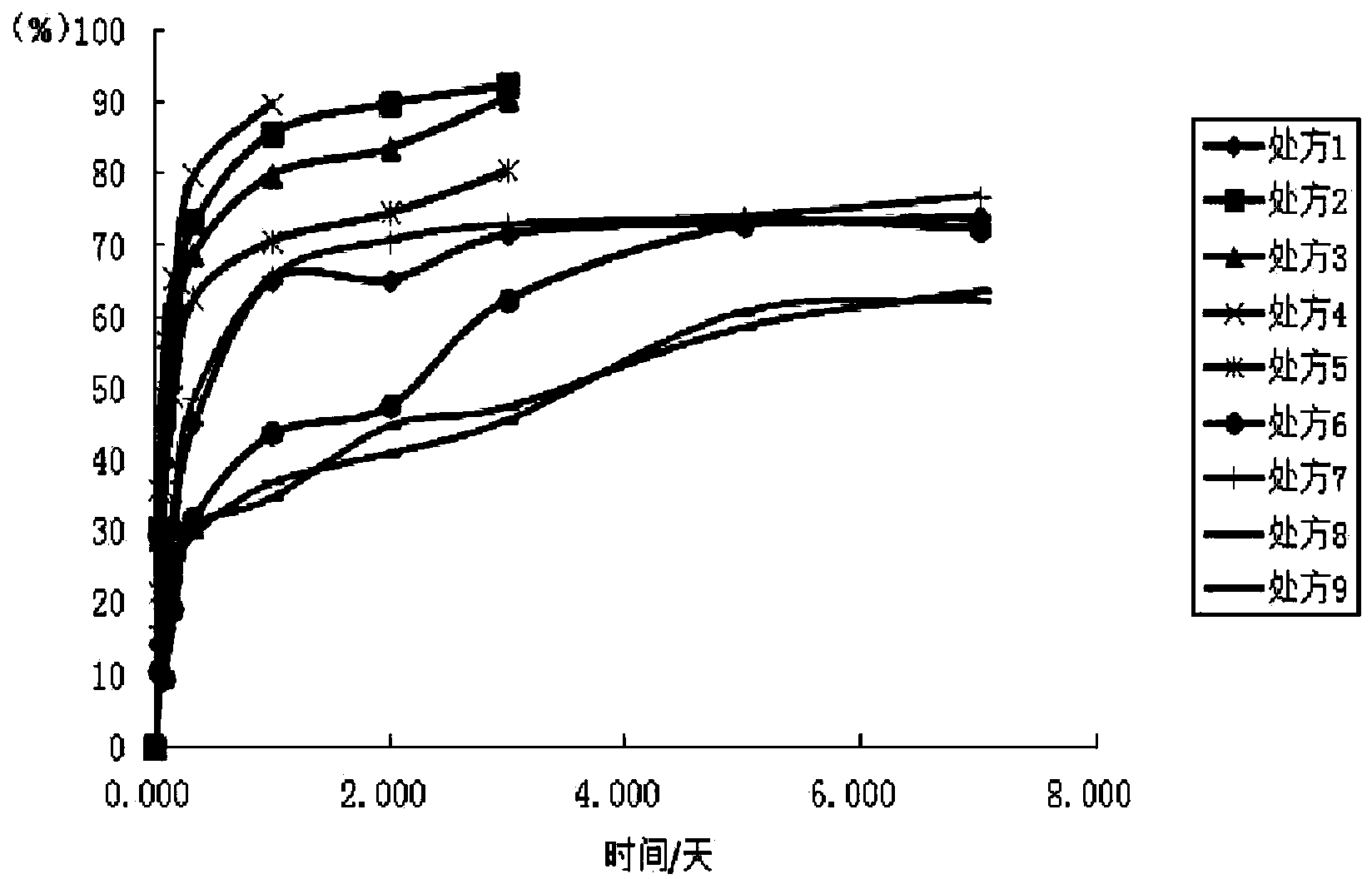
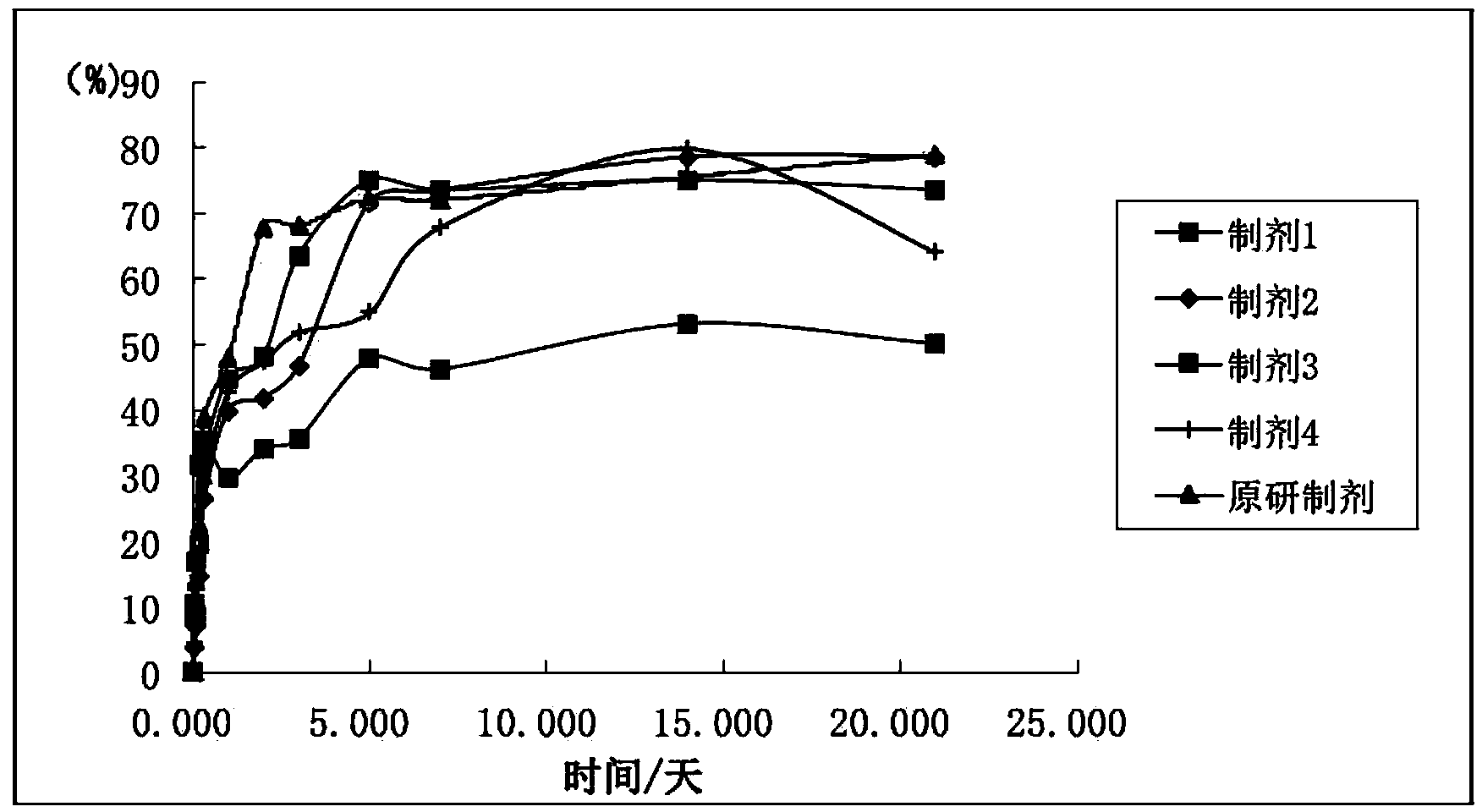
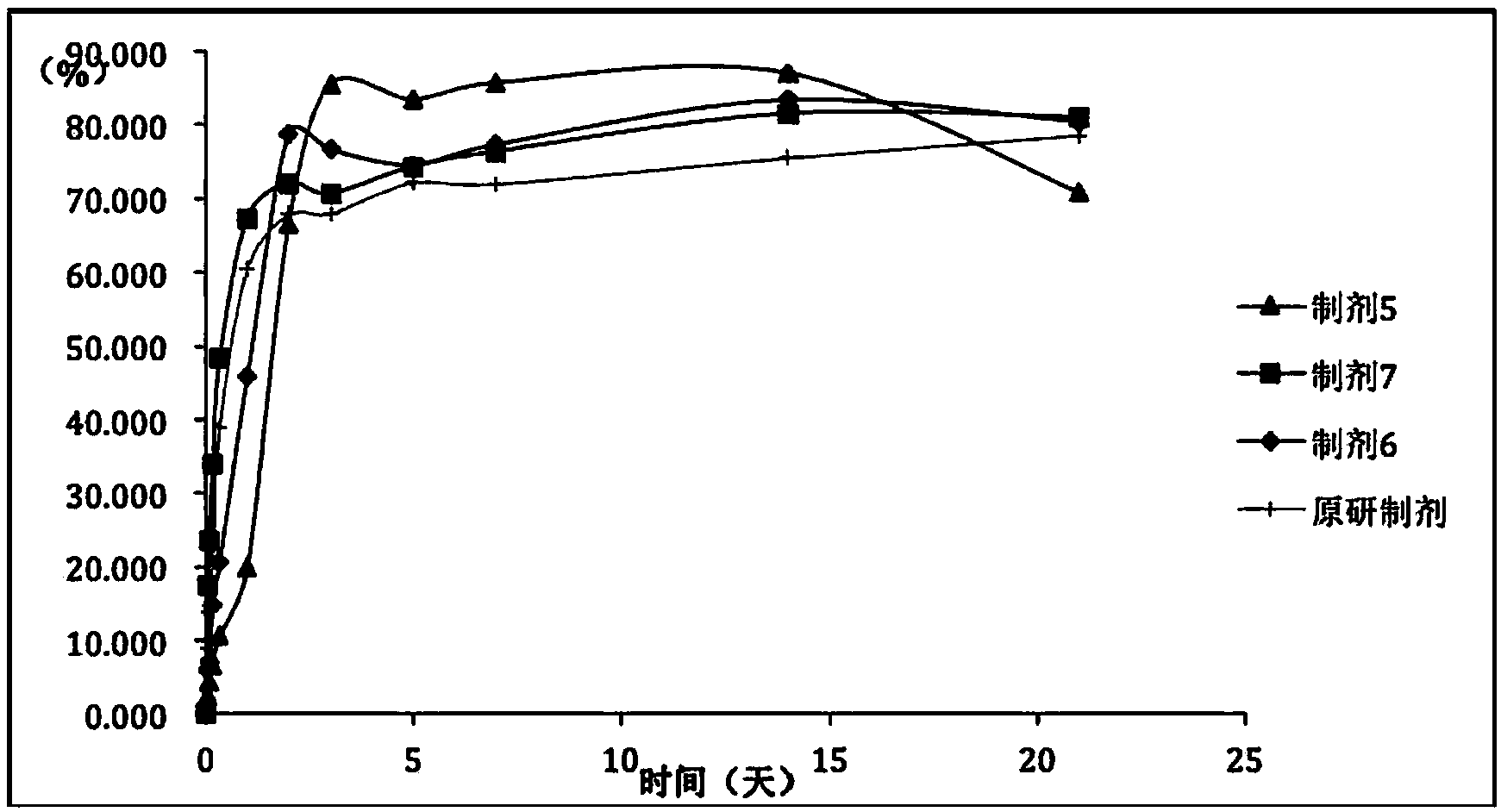
[0058] As can be seen from the above tests, prescription 1 and prescription 7 have very similar release profiles in vitro ( figure 1 ). Fulvestrant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com