Thyroid peroxidase antibody magnetic-particle chemiluminescence immune quantitative testing kit
A chemiluminescence immunoassay and peroxidase technology, which is applied in the field of immunoassay medicine, can solve the problems of low sensitivity of the enzyme-linked immunosorbent assay, no fully automated operation, poor repeatability of measured values, etc., and achieves good specificity, low cost, and detection. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of TPO-Ab Magnetic Particle Chemiluminescent Immunoquantitative Assay Kit
[0035] (1) Preparation of TPO-Ab calibrator:
[0036] The TPO-Ab calibrator diluent is configured into a calibrator concentrated stock solution, the calibrator diluent is prepared with a phosphate buffer solution containing 1% BSA, calibrated with an enterprise calibrator, and the concentrated stock solution Dilute to the working concentration with calibrator diluent, respectively 0, 10, 25, 100, 200, 500IU / mL;
[0037] (2) Preparation of TPO-Ab quality control products:
[0038] Dilute the above concentrated stock solution to 18IU / mL and 350IU / mL with calibrator diluent, 18IU / mL is the low-value quality control, and 350IU / mL is the high-value quality control;
[0039] (3) Preparation of biotin-labeled TPO-b
[0040] Take 0.5mg of TPO antigen, and dialyze with borate buffer solution at 2-8°C for 1-3h; add 45ug of biotin to the dialyzed antigen, and add dimethyl sulfoxid...
Embodiment 2
[0049] Embodiment 2: the inspection of kit of the present invention
[0050] (1) Physical inspection: liquid components should be clear without sediment or floc; other components should have no package damage.
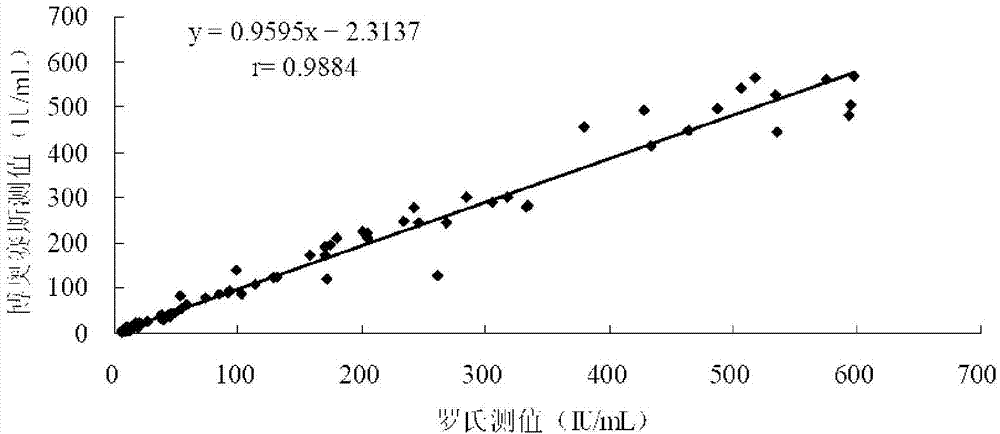
[0051] (2) Accuracy: The calibration product of the kit and the national standard product series are analyzed and measured at the same time, and the double logarithmic mathematical model is used for fitting, requiring that the two dose-response curves do not deviate significantly from parallel (t test, |t|<2.447) ; Take the TPO-Ab enterprise standard as the reference substance, and use the double-logarithmic mathematical model to fit. The average value of the ratio between the measured value and the labeled value of the kit calibrator should be within the range of 0.90 to 1.10.
[0052] (3) Linearity of the dose-response curve: Fitting with a double-logarithmic mathematical model, the absolute value of the correlation coefficient r of the dose-response curve within the...
Embodiment 3
[0060] Embodiment 3: the using method of kit of the present invention
[0061] (1) Equilibrate the test kit at room temperature (18-25°C) for 30 minutes.
[0062] (2) Preparation of lotion: Dilute the concentrated lotion with distilled water at 1:20 (1mL of lotion plus 19mL of distilled water). If the concentrated lotion has crystals, the concentrated lotion can be placed at room temperature or 37°C, and then diluted after the crystals dissolve.
[0063] (3) Number the reaction tube, add 25uL calibrator or serum sample, 50uL magnetic particle-streptavidin suspension, 75uL biotin-TPO-Ag conjugate, 100uL mouse anti-human enzyme conjugate, Shake the reaction at 37°C for 15 minutes, place the test tube rack on the magnetic separator for separation for 2 minutes, then pour out the supernatant, add 500uL washing solution, mix well, separate on the magnetic separator, pour out the washing solution, repeat 3 times Next, add 50uL of chemiluminescence substrate solution A and 50uL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com