Immobilized bichiral ligand metal complex and synthetic method and application thereof
A metal complex, chiral technology, applied in organic chemistry methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as high cost, inability to use hydrogen peroxide, fire, etc. Achieve the effect of easy regeneration, simple and easy immobilization method, and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1. Preparation of L-type immobilized bimanual ligand
[0038] Soak 50 grams of D311 macroporous anion exchange resin with 500ml 1mol / L NaOH overnight, filter, and wash thoroughly with purified water to pH 7.5, 80 0 Dry at C for 5 hours, take 1 gram of dry resin, and measure its exchange equivalent to 7.5 mmol / g.
[0039] Add 10g of the above dry D311 resin, 50ml of toluene, 90mmol L-type methyl mandelic acid (14.9g) into the reaction flask, heat it to reflux at 110°C for 10 hours, cool to room temperature, filter, and wash with 150ml of toluene. 0 C was dried for 5 hours to obtain 20 g of L-type immobilized bimanual ligand, the content of L-type mandelic acid amide unit was 3.85 mmol / g. The structural formula of the L-type immobilized bimanual ligand is:
[0040]
example 2
[0041] Example 2. Preparation of D-type immobilized bimanual ligand
[0042] Add 10 grams of the above dry D311 resin, 50ml xylene, 80mmol D-mandelic acid methyl ester (13.3g) into the reaction flask, heat it to reflux at 120°C for 5 hours, cool to room temperature, filter, wash with 150ml xylene, 90 0 C was dried for 5 hours to obtain 20 g of D-type immobilized bimanual ligand, the content of D-mandelic acid amide unit was 3.85 mmol / g. The structural formula of the L-type immobilized bimanual ligand is:
[0043]
[0044] 2. Preparation of immobilized bimanual ligand metal complex
[0045] Example 1: Preparation of L-type or D-type immobilized bimanual ligand titanium complex
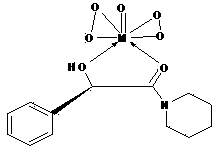
[0046] Add 20g of L-type or D-type immobilized bimanual ligand, 100ml of chlorobenzene, 38.5mmol (10.9g) of isopropyl titanate, 85 to the reaction flask 0 C react for 2 hours, cool to room temperature, filter, wash with 50ml methanol, 70 0 C was dried for 5 hours to obtain 26.3 grams of L-type or D-type i...
example 3
[0055] Example 3. Preparation of L-type or D-type immobilized two-handed ligand tungsten complex
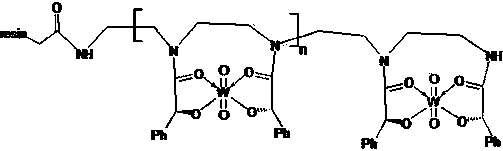
[0056] Add 20g of L-type or D-type immobilized bimanual ligand, 100ml of isopropanol, 3g of triethylamine to the reaction flask, and cool to 0 0 C, 38.5mmol (14.6g) of tungsten hexachloride solution (50ml) in chloroform was added dropwise to control the temperature of 10-15 0 C, slowly warm up to 45 after dropping 0 C react for 2 hours, cool to room temperature, add 5ml water, stir for half an hour, filter, wash with 50ml isopropanol, 50ml 0 C was dried for 8 hours to obtain 28.2 grams of L-type or D-type immobilized bimanual ligand tungsten complex. The structural formula of the L-type immobilized bimanual ligand tungsten complex is:
[0057]
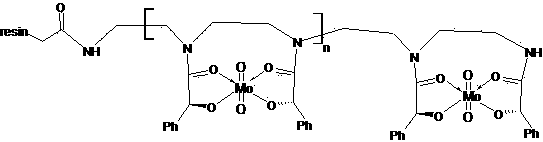
[0058] The structural formula of D-type immobilized bimanual ligand tungsten complex is:
[0059]
[0060] The chiral ligand unit (L-type or D-mandelic acid amide unit) of the immobilized bimanual ligand forms a complex with a molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com