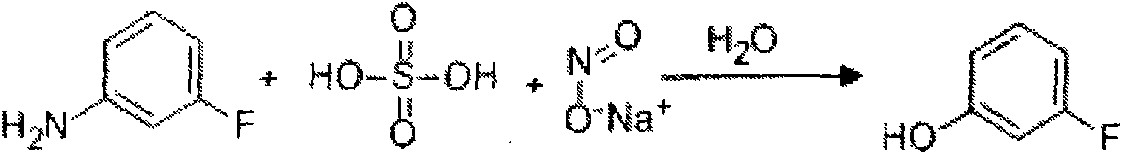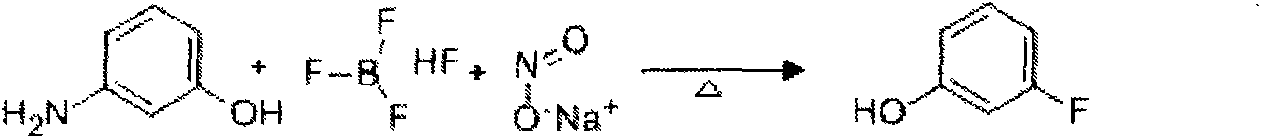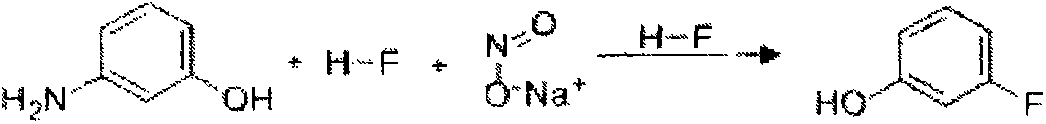Preparation method of 3-fluorophenol
A technology of fluorophenol and aminophenol, which is applied in the field of preparation of 3-fluorophenol, can solve the problems of unsuitability for industrial production, high technical difficulty, and rare raw materials, and achieve the effects of low environmental protection pressure, simple process equipment, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) 1000L equipped with frequency conversion stirring, solvent metering tank, alkali solution dripping tank, reflux condenser, temperature display gauge, screw conveyor, add 600L of toluene into the lead-lined reaction kettle, start stirring, and add it evenly by the screw conveyor at room temperature The raw material m-aminophenol is 200kg, and the speed of the reactor is gradually increased to 100red / min. After all the raw materials are dissolved, the reactor is sealed, and the jacketed brine cooling valve of the reflux condenser is opened, and the anhydrous hydrofluoric acid metering tank is dripped into the reactor. Add 110kg of anhydrous hydrofluoric acid, and control the reaction temperature to be 20-25°C. After the anhydrous hydrofluoric acid was added dropwise, the reaction was incubated for 60 minutes.
[0044] (2) Open the cooling brine valve of the jacket of the reaction kettle, and after the temperature in the reaction kettle reaches -10°C, add KNO evenly fr...
Embodiment 2
[0050] (1) 1000L is equipped with frequency conversion stirring, solvent metering tank, alkali solution dripping tank, reflux condenser, temperature indicator, lead-lined reaction kettle of screw conveyor, add 600L of fluorobenzene into the reaction kettle, start stirring, and the screw conveyor is evenly distributed at room temperature Add 200kg of raw material m-aminophenol, and gradually increase the speed of the reactor to 100red / min. After all the raw materials are dissolved, close the reactor, open the jacketed brine cooling valve of the reflux condenser, and flow from the anhydrous hydrofluoric acid metering tank to the reactor. 120kg of anhydrous hydrofluoric acid was added dropwise to the mixture, and the reaction temperature was controlled to be 20-25°C. After the anhydrous hydrofluoric acid was added dropwise, the reaction was incubated for 60 minutes.
[0051] (2) Open the cooling brine valve of the jacket of the reaction kettle, and after the temperature in the re...
Embodiment 3
[0057] (1) 1000L is equipped with frequency conversion stirring, solvent metering tank, alkali solution dripping tank, reflux condenser, temperature display gauge, lead-lined reaction kettle with screw conveyor, add m-fluorotoluene 600L, start stirring, and the screw conveyor Evenly add 200kg of raw material m-aminophenol, and gradually increase the speed of the reactor to 100red / min. After all the raw materials are dissolved, seal the reactor, open the jacketed brine cooling valve of the reflux condenser, and flow from the anhydrous hydrofluoric acid metering tank to the reactor. 115kg of anhydrous hydrofluoric acid was added dropwise to the mixture, and the reaction temperature was controlled to be 20-25°C. After the anhydrous hydrofluoric acid was added dropwise, the reaction was incubated for 60 minutes.
[0058] (2) Open the cooling brine valve of the jacket of the reaction kettle, and after the temperature in the reaction kettle reaches -10°C, add KNO evenly from the scr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com